More Information
Submitted: April 21, 2025 | Approved: May 28, 2025 | Published: May 29, 2025
How to cite this article: Rokyta R, Holeček V, Fricova J. Free Radicals, Antioxidants and Redox Potential. Heighpubs Otolaryngol Rhinol. 2025; 9(1): 005-010. Available from:
https://dx.doi.org/10.29328/journal.hor.1001031.
DOI: 10.29328/journal.hor.1001031
Copyright license: © 2025 Rokyta R, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Free radicals; Antioxidants; Mutations; Diseases; Oxidative stress; Aging; Malondialdehyde; Oxido-reductive potentials
Free Radicals, Antioxidants and Redox Potential
Rokyta R1*, Holeček V2 and Fricova J3
1Department of Physiology, Third Faculty of Medicine, Charles University, Prague, Czech Republic
2Department of Clinical Biochemistry, Mulac Hospital, Pilsen, Czech Republic
2Department of Pain Management, Charles University, First Faculty of Medecine and General University Hospital, Czech Republic
*Address for Correspondence: Rokyta R, Department of Physiology, Third Faculty of Medicine, Charles University, Prague, Czech Republic, Email: [email protected]
The role of free radicals and antioxidants is often underestimated despite their involvement in key metabolic processes, although they participate in many important metabolic processes in the life of humans, animals and plants. Their quantity and quality differ from each other, which is not respected. Each cell is attacked approximately 10,000 times by free radicals. Oxidative stress is the cause of many problems, especially in viral diseases. Monitoring of redox potentials in body fluids is usually not carried out. Viral replication is influenced by oxidative energy, derived from either host metabolism or free radical activity, which is supplied by oxidation by free radicals or the host. Nucleic acid mutations due to the effect of free radicals can be the cause of carcinomas, and possible defense against mutations could help eradicate dangerous viruses. The importance of malondialdehyde and antibodies against it is discussed. Eliminating free radicals, reducing lipoperoxidation, and protecting against environmental oxidative stress are important factors for human health.
Study objective: To highlight the importance of free radicals, antioxidants and redox potentials for patient diagnosis and therapy.
Methods: This study synthesizes findings from multiple published sources, including our own research.
Results: Findings highlight the role of free radicals in oxidative stress, DNA damage, and viral replication, with redox potential (ORP) and Malondialdehyde (MDA) identified as key diagnostic markers.
Conclusion: Monitoring oxidative balance and targeting free radical activity are essential for preventing cellular damage and improving clinical outcomes in oxidative stress-related diseases.
One of the basic values of human homeostasis is the maintenance of redox states. We are constantly breathing, our hearts are beating, we are consuming energy. To do this, it is necessary to maintain the internal environment, including redox potentials. Redox potential homeostasis is maintained by the ratio of free radicals and antioxidants. Burning is a source of energy, burning is oxidation, but for research, the fact that oxidation involves the loss of an electron. Is more important. Reduction (antioxidation) is then the gain of an electron.
Oxidative stress and redox potentials
Monitoring of the redox state is currently done little or only indirectly (total antioxidant capacity, NAD+/NADH quotient, ABR). The resulting ratio can probably best be monitored by the so-called redox potential (ORP). This is measured using two electrodes (e.g. platinum and calomel) connected with a saturated KCl solution and a voltmeter to measure the difference in potential between the two electrodes. A positive value means pro-oxidative, a negative value means antioxidant potential. An excess of free radicals is oxidative stress, an excess of antioxidants is reductive stress. Oxidative stress is more common. The body is attacked daily by a large number of radicals from both the external and internal environment.
Mechanisms of damage
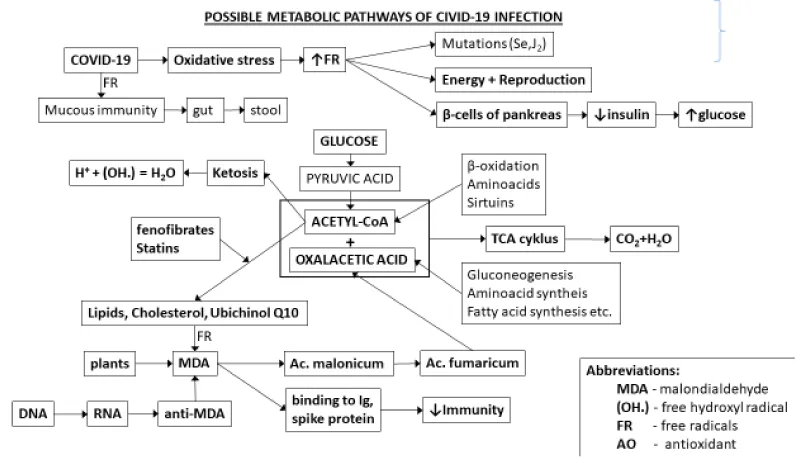
Free radicals oxidize carbohydrates and fats (lipoperoxidation produces carcinogenic aldehydes 4-hydroxynonenal and malondialdehyde [1,2]. Malondialdehyde (CH2(CHO)2) and other aldehydes change the structure and function of the lungs by binding to proteins [3]. (MDA) after oxidation by free radicals to malonic acid (CH2(COOH)2) can bind several proteins by peptide bonds through the reaction of carboxyl groups with free amino groups of proteins. A complex molecule is formed that the organism does not recognize and antibodies are formed against it, which are degraded in proteasomes [4]. Immunoglobulins or spike proteins of vaccines can be neutralized in this way. Viruses are thus protected from death. Malondialdehyde and related aldehydes alter lung structure and function by binding to proteins, particularly surfactant protein D (SPD). However, MDA is also formed by the effect of cyclooxygenase on membrane proteins, or we absorb it from plants, which use MDA to protect themselves from insects or herbivores. Redox imbalance in viral infections is involved in cell death.
Viral pathogenesis and mutations
RSV (Respiratory Syncytial Virus) increases lipoperoxidation, reduces glutathione levels, activates pro-inflammatory cytokines and inhibits Nrf2. On the contrary, humans defend themselves by producing anti-MDA. The hope is the epigenetic emergence of genetic anti-MDA defense in human DNA: anti-MDA → RNA → DNA), the consequences of oxidative modification of proteins are irreversible and negatively affect the physiological functions of proteins. There may be disturbances in their enzymatic, structural and binding properties, induction of apoptosis or necrosis. The accumulation of damaged, non-degradable proteins can lead to complete inhibition of proteasomes. In proteins, their glycation and subsequent changes change their physical and chemical properties (carbonyls, etc. AGE- advanced glycation end-products) [1]. They include, for example: change in solubility, charge and isoelectric point, crosslinking of chains, increased resistance to thermal denaturation and stability to pH reduction) and nucleic acids (oxidation of nitrogenous bases causes mutations - e.g. adenine binds thymine, oxidized adenine then cytosine). Mutations also occur in RNA-dependent RNA polymerase (RdRp), which introduces errors into the virus genome during its replication. RNA is more susceptible to oxidative damage. These infections cannot be eradicated without removing mutations. Mutations can probably be prevented by small molecules that get close to nucleic acids and are preferentially oxidized before nitrogenous bases by free radicals, e.g.selenite (perhaps also potassium iodide).
Se4+ -2 e- →Se6+ [5].
Sources and roles of free radicals
The presence of free radicals in the air is constantly increasing with technological development. There are more and more pollutants in the air, but free radicals are caused by UV rays, radioactivity (2,000 mSv of radiation is fatal), X-rays, cosmic rays, atomic explosions, smoking (1 cigarette represents 1017 free radicals), but humans themselves also produce free radicals (in the reperfusion phase after ischemia, oxidation of purines, etc.). They are needed to kill tumor cells (NK cells), microbes (leukocytes, pus), fungi, yeasts, parasites and for signaling to the brain (hunger, cold, etc.).
Essentially all pathological conditions are accompanied by the production of free radicals, which act as their cause or accompanying phenomenon. In any case, this is a complicating factor. Free radicals vary in how quickly they react to regain stability; the hydroxyl radical is among the most reactive, probably the strongest free radical is the free hydroxyl radical, half of which lasts in the body for only 10-9 seconds. Free radicals are also produced to an increased extent during physical activity, after administration of certain drugs, they can be caused by a change in diet, alcohol consumption. It seems that we age as a result of the constant intracellular production of free radicals that damage tissues, these [6] mutations also support the formation of tumors [7]. Already formed tumors are removed by surgery and chemotherapy, paradoxically also free radicals (cis-platinum, adriamycin, X-ray irradiation, etc.). Healing occurs only after the removal of tumor-initiated stem cells.
Energy metabolism and mitochondrial dynamics
In history, humanity has suffered more from hunger, and only recently from an excess of food. So humans tend to protect their energy reserves. Anaerobic oxidation of glucose produces lactic acid, from which the key substance acetyl-CoA is formed. When life is threatened, the level of sirtuins (histone deacetylators) increases. Acetyl-CoA is metabolized into higher fatty acids, cholesterol and ubiquinone Q10. Acetyl-CoA gives with oxaloacetic acid isocitric acid and is burned in the tricarboxylic acid cycle to CO2 and H2O to form energy-rich substances, oxaloacetic acid is regenerated. Acetyl-CoA can further be metabolized to acetone, acetoacetic acid and β-hydroxybutyric acid. Ketonemia is used for weight loss (Table 1).
Table 1:
Protective role of antioxidants
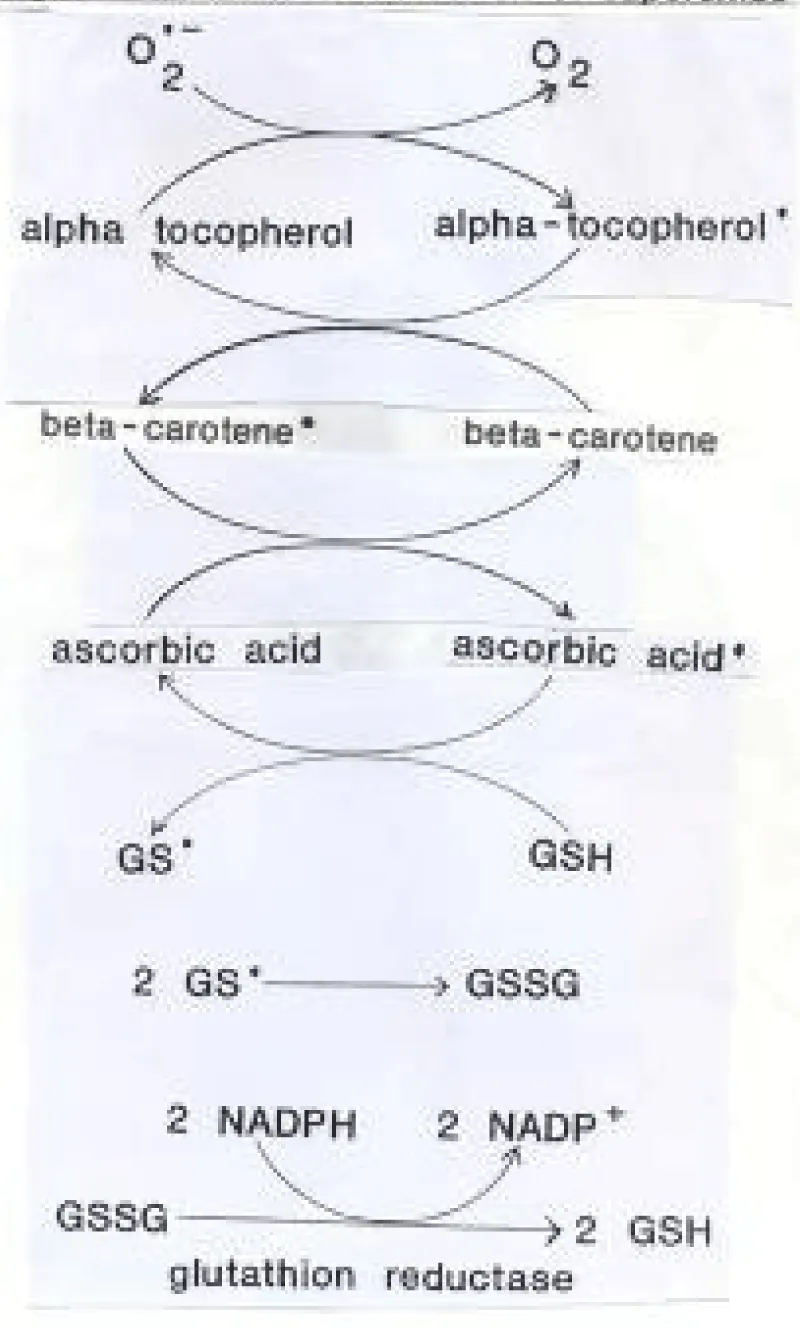
Antioxidants, by releasing electrons, replenish the unpaired electrons of free radicals with paired electrons and thus eliminate them. However, by losing an electron, antioxidants themselves become free radicals (loss of an electron is oxidation, gain of an electron is reduction or antioxidation). Substances with a higher redox potential oxidize substances with a lower potential, but not vice versa. Termination (quenching) of the free radical cascade occurs, for example, by the effect of molecular hydrogen, glutathione, glutathione reductase, NADPH or by the reaction of two free radicals that share their unpaired electrons. An example is the removal of superoxide by a cascade of electron transfers (this mechanism can perhaps be used in signaling): (image taken from the Internet). The rapid transfer of electrons can create a current that could perhaps bridge a traumatically severed nerve (Figure 1).
Figure 1:
Antioxidants in disease prevention
The antioxidant Molecular hydrogen shows potential therapeutic benefits in various oxidative stress-related diseases, such as inflammation, cardiovascular diseases, obesity, hemodialysis, after radiation, neurodegenerative and oncological diseases, type 2 diabetes, psoriasis, fatigue and post-covid syndrome, etc [8].
By excreting H+ in feces, saliva, sweat, intestinal gases, etc., and by breathing CO2, the organism saves electrons, but loses the ability to obtain energy from the excreted products.
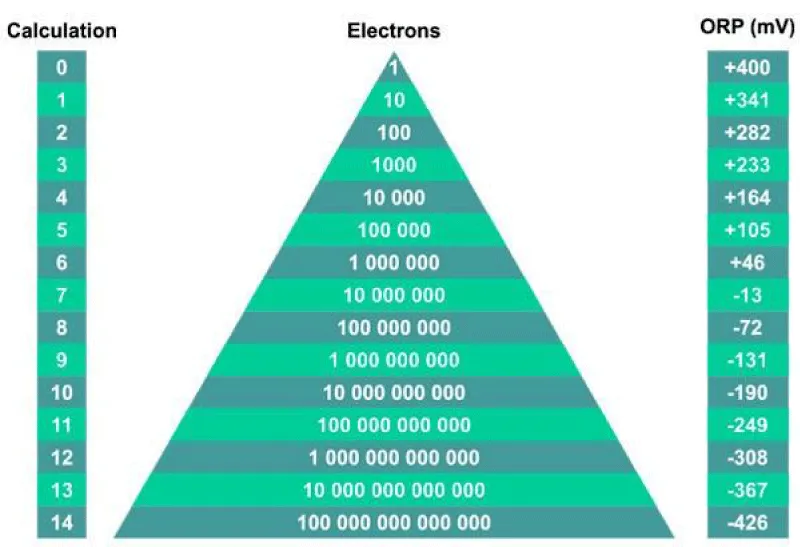
The oxidation-reduction potential depends on the pH and the number of electrons. The normal pH of blood is 7.36 -7.44, i.e. in a slightly alkaline state. A higher amount of OH- groups compared to H+ means that there is a slight excess of electrons in the blood needed for immediate oxidation (i.e. the addition of an unpaired electron to a paired one) of free radicals. Blood circulation allows tissue repair by supplying electrons. At pH = 0, ORP is around + 425 mV, at pH 7 it approaches zero, pH 14 is around -425 mV. The average pH of human blood is 7.4, the antioxidant potential, after circulation, decreases slightly, which means that the blood supplies electrons to repair oxidatively damaged tissues (Figure 2).
Figure 2: The oxidation-reduction potential depends on the pH and the number of electrons.
Redox potential in food and water
Oxidation-reduction potential (The ORP (or pH) of drinking water (tap water) is usually greater than zero, and ranges from +180 mV to +300 mV. The difference in ORP between the internal environment and drinking water means that the body’s energy must be spent on correcting the activity of the water’s electrons. Therefore, the lower the ORP of the water, the more free electrons there are and the greater the antioxidant capacity. Hydration of cells and tissues is facilitated. If the drinking water that enters the body has an ORP close to the ORP value of the internal environment of the human body, the electrical energy of the cell membranes is not spent on correcting the activity of the water electrons, and the water is immediately digested because it is compatible with the ORP of the tissues. If oxidative processes begin to prevail over recovery processes, the defenses and functions of vital organs begin to weaken and they are no longer able to independently resist various diseases.
The ORP of meat decreases after slaughter from about +250 mV during meat maturation to values of -200 mV. Foods of plant origin (e.g. fruit and vegetable juices) have high ORP values (+300 to +400 mV). Atmospheric oxygen maintains aerobic conditions and a high redox potential in the surface layers of the food (about a few mm), even in liquid products, by diffusion. The deeper the layer, the lower the redox potential.
The predominance of free radicals over antioxidants is called oxidative stress, the reverse imbalance is called reductive stress [9-11]. (Weech, 2020). At pH 7 it approaches zero, pH 14 (the greatest excess of OH- ions) is around -425 mV. The average human pH is 7.4, so the antioxidant potential, after circulation, decreases slightly, which means that the blood supplies electrons to repair oxidatively damaged tissues.
The redox potential of the human internal environment ranges between -100 mV and -200 mV, i.e. in an antioxidant environment. This means that antioxidants, or OH- ions, predominate and the organism protects itself with enough electrons. Antioxidants with a higher redox potential oxidize those with a lower redox potential, but not vice versa. Proteins as antioxidants capture 50% - 75% of all free radicals formed.
The ORP of foods and beverages also plays an important role. The ORP of raw milk is usually in the range of +200 to +300 mV, this value is influenced mainly by the amount of dissolved oxygen. Heat treatment reduces the redox potential of milk to +120 mV, as a result of protein denaturation, which exposes reducing sulfhydryl and disulfide groups and creates other reducing substances. The ORP of milk is also reduced by the activity of bacteria - they consume oxygen and produce reducing metabolic products.
Clinical implications and research gaps
Energetics, free radicals and antioxidants are coming to the forefront of interest, but clinical biochemistry has not yet responded adequately to this. There are many unresolved issues, some of which we would like to draw attention to. The physiological range of pro- and antioxidant potential of tissues and body fluids is still unknown, even in diseases. Oxidative stress, which accompanies many diseases including infectious [5] and conditions, has its reason, and not only as a source of energy. Oxidation produces energy, but it also damages tissues, which forces the body to repair the damage. With aging, this ability, as well as the overall antioxidant capacity, decreases.
Oxidative stress has been described, for example, in viral diseases [5] hepatitis, in infertile men, neurodegenerative diseases [13,14] such as Alzheimer’s disease, Parkinson’s disease, but perhaps also in others (migraine, Hutchinson’s disease, amyotrophic lateral sclerosis, multiple sclerosis, schizophrenia, sickle cell anemia, etc.), where it is not commonly known. Examination of the redox potential in cerebrospinal fluid could be useful.
The most common cause of blindness in old age is macular degeneration, where oxidative stress also appears to be the cause, and other skin diseases such as ulcus cruris, melanoma, alopecia, etc. should be examined by redox potential. Smoking is accompanied by pro-oxidative potential, perhaps a quantitative examination would reveal a dangerous level for the development of lung cancer. Free radicals can seriously damage protein molecules and disrupt vital processes in sperm and thus its ability to fertilize an egg. “Therefore, sperm produce sulfane, which binds to specific ‘vulnerable’ sites of proteins in sperm, thereby protecting them from damage. Vitamin E is believed to play a crucial role in protecting sperm membranes, which are very susceptible to damage by oxidative stress. Vitamin E also has anti-inflammatory effects and is therefore able to reduce oxidative stress caused by leukocytes. 200 mg of vitamin E per day significantly reduced MDA levels in semen after just one month of use and improved the fertilization potential of these sperm. Transfusion blood cans, where free radicals are also produced, would probably improve their quality by adding antioxidants to the can or by applying them to the donor before collection. Humans can produce antioxidants in their own bodies (uric acid, bilirubin, etc.).
Free radicals accumulate in the brain during the day, and at night, during sleep and darkness, they are released into the circulation. The brain consumes 20% of the oxygen inhaled and various forms of hypoxia affect ORP [12]. It is questionable whether free radicals are involved in the formation of tau protein and β-amyloid by damaging proteins in the brain.
A newborn baby has a higher bilirubin level after birth because their body has not yet developed antioxidant protection against oxygen. The administration of antioxidants in hyperbilirubinemia has probably not been tested yet. Free radicals in the intestinal tract can damage the mucosa and the production of immunoglobulins. It would be interesting to monitor patients with gastric and intestinal carcinoma to see if a regular daily supply of antioxidants to the p. os will not affect the incidence or course of the disease [15]. An interesting idea is to apply the herpes virus to the tumor. Oxidative stress from herpes viruses causes the splitting of biomolecules in the tumor, thus increasing the intracellular osmotic pressure until the cell ruptures and its contents are flushed into the intercellular space. The virus enters other cells through ACE receptors and the tumor shrinks. The removal of fats by oxidation (pro-oxidative state) indicates the possibility of weight loss, an antioxidant state that prevents oxidation helps to maintain or increase weight. Antioxidants are used in the transport of transplanted organs, their use for pain relief has not yet been used. In myocardial infarction (ischemia and reperfusion), humans produce antioxidants (nicotinamide mononucleotide - NMN), it would probably be useful to increase antioxidant defenses [16]. Free radicals oxidize LDL-cholesterol, which enters cells, where it activates proteases (collagenase, elastase), foam cells are formed, the basis of atherosclerosis, antioxidants prevent the oxidation of LDL-cholesterol.
After a certain period of time, also due to the influence of free radicals, the cell enters a state of senescence, when it loses its ability to proliferate. This process can be slowed down by the effect of antioxidants [17]. In old age, the immune system fails to eliminate senescent cells, which can disrupt tissue function and cause tumors. These senescent or old cells exhaust the organism. In old age, the immune system fails to eliminate senescent cells. These senescent or old cells exhaust the organism. They still take nutrients, need oxygen, but do not produce anything important. Pycnogenol, quercetin, fisetin, pterostilbene and L-theanine from green tea are used as senolytic agents. In lymphedema, the antioxidant capacity is reduced, in osteoporosis, but free radicals and antioxidants are apparently also involved in bone remodeling. In heavy work and top-level sports, free radicals are produced, which increase insulin sensitivity. Therefore, immediately after the exercise, an ice bath is recommended to slow down the formation of free radicals and the administration of antioxidants such as glutathione [18], so that the tissues are more protected. The white fibers in the legs are fast and sensitive to free radicals, while the red fibers are more durable and resistant.
Mitochondrial health is influenced not only by our diet, but also by exercise, sleep and stress management. Research shows that bioactive substances such as fiber and polyphenols can support mitochondrial health. These substances support the formation of brown adipose tissue, which, unlike white fat, burns calories and thus helps us maintain a healthy weight.
A healthy microbiome, the community of microorganisms in the gut, produces a number of beneficial metabolites that help burn calories. However, if our microbiome is disrupted (for example, due to stress, antibiotics, or the consumption of processed foods), our body may struggle to burn calories efficiently.
Administration of certain antioxidants can significantly affect human health
The antioxidant molecular hydrogen increases mental alertness and provides energy to the body. It is used for inflammation, cardiovascular diseases after radiation, neurodegenerative and oncological diseases, type 2 diabetes, psoriasis, fatigue and post-COVID syndrome, etc [10].
Melatonin: It reduces the absorption of cholesterol, especially its LDL fraction, also reduces the levels of triacylglycerols, increases the level of HDL. It regulates blood flow through the brain [18].
Pyridine coenzymes (NMNH, NADH - nicotinamide adenine dinucleotide, NADPH). The biosynthesis of pyridine coenzymes starts from nicotinic acid and nicotinamide [17]. A secondary pathway of NAD synthesis produces serotonin and melatonin [11]. NMN is a precursor of NAD+ and reduces edema and brain damage as well as hematoma size, oxidative stress and inflammation. A decrease in NAD+ in mitochondria is considered a cause of aging and memory loss. Administration of NAD+ protects against brain disorders such as amylotrophic lateral sclerosis or stroke. Low NAD+ levels disrupt communication between the brain and mitochondria. At the age of 80, people tend to have only 1% - 10% of their youth levels. NAD+ increases after resveratrol [17], exercise and low calorie intake. If we did not have NAD+ in our bodies, we would die within 30 seconds.
Taurine: It is crucial for supporting healthy metabolism, protecting organs, and contributing to the retention of information in long-term memory.
Ergothioneine is an amino acid, its levels decrease with age. A protein carrier moves ergothioneine into cells, especially those that face high levels of oxidative stress, such as red blood cells and cells in the central nervous system.
Glutathione is an intracellular antioxidant [5,12]. In newly hospitalized patients with chronic diseases, there is a deficiency of reduced glutathione in the blood cells. Conversely, in people with excellent physical condition and good mental health, the level is high [5]. Its production can be increased by cysteine deficiency (e.g. ACC).
Thioredoxin reductase: Important intracellular antioxidant, selenoenzyme. It is important for DNA synthesis, fertilization, and acts against oxidative stress in the CNS. It protects Intracellular antioxidants.
Ginkgo biloba: Extract from the leaves of the ginkgo biloba tree. Contains flavone glycosides, increases resistance to low oxygen levels, improves blood circulation in the brain (reduces blood viscosity), reduces fatigue, improves sexual function. Protects against lipoperoxidation, inhibits thromboxane synthesis.
Coenzyme Q10 (Ubiquinol)
Among people over 90 years old, those who had higher levels of coenzyme Q10 in their blood, which protects brain cells, were significantly more mentally capable.
Fenofibrate: Fenofibrate has been proposed as a potential therapeutic agent in COVID-19 due to its anti-inflammatory and metabolic effects [9].
Flavonoids: They are scavengers of peroxyl and hydroxyl free radicals, protect against lipoperoxidation, inhibit lipoxygenase and cyclooxygenase, chelators of metal ions. There are more than 5000 of them, most of them are of plant origin.
Apigenin is an antioxidant and anti-inflammatory compound that occurs naturally in plants. It is found in fruits and vegetables.
Resveratrole: Antioxidant from red wine. Improves mood, helps against insulin resistance.
NRF2 (Nrf2 is a nuclear erythroid 2-related factor, a regulator of resistance to oxidants). It has a central role in defense mechanisms against oxidative and electrophilic damage. It is a transcription factor that represents the protection of cells from oxidative, proteotoxic and metabolic stress. It plays a key role in protecting the organism against stress.
Sirtuins: There are 7 NAD+-dependent histone deacetylase enzymes (SIRT1 – SIRT7). Their production is stimulated by life-threatening events, stress, caloric restriction, and intense exercise. They have been shown in animals to extend lifespan. Inhibitors of histone deacetylases - sirtuins (apicidin, trichostatin-a, panobinostat, and entinostat) have neuroprotective and neurodegenerative properties.
Selenium: Its deficiency causes cardiomyopathy. High doses of selenium go to the muscles, some organs have a higher metabolic priority for selenium, e.g. the testes. While the bioavailability of transit metals rarely exceeds 20%, dietary selenium (both organic and inorganic) is absorbed preferentially by 60% or more, in some cases even 100%. We find many new antioxidants in the tropics and seaweed.
The presented evidence highlights the critical role of redox processes and underscores the need for more systematic monitoring and their monitoring deserves more attention than has been paid to them so far.
- Muralidharan A, Bauer C, Katafiasz DM, Oyewole OO, Morwitzer MJ, Roy E, et al. Malondialdehyde acetaldehyde adduction of surfactant protein D attenuates SARS-CoV-2 spike protein binding and virus neutralization. Alcohol Clin Exp Res. 2023;47(1):95–103. Available from: https://doi.org/10.1111/acer.14974
- Martín-Fernández M, Aller R, Heredia-Rodríguez M, Gómez-Sánchez E, Martínez-Paz P, Gonzalo-Benito H, et al. Lipid peroxidation as a hallmark of severity in COVID-19 patients. Redox Biol. 2021;48:102181. Available from: https://doi.org/10.1016/j.redox.2021.102181
- Pláteník J. Free radicals, antioxidants and aging. Intern Med (Czech Republic). 2009;11(1):30–3. Available from: https://www.internimedicina.cz/pdfs/int/2009/01/06.pdf
- Nair CL, O'Neil P, Wang G. Malondialdehyde. In: Encyclopedia of Reagents for Organic Synthesis. New York: John Wiley & Sons; 2008. Available from: https://doi.org/10.1002/047084289X.rm013.pub2
- Ebrahimi M, Norouzi P, Aazami H, Moosavi-Movahedi AA. Review on oxidative stress relation on COVID-19: Biomolecular and bioanalytical approach. Int J Biol Macromol. 2021;189:802–18. Available from: https://doi.org/10.1016/j.ijbiomac.2021.08.095
- Hiffler L, Rakotoambinina B. Selenium deficiency promotes mutations, replication, and virulence of RNA viruses, and selenium has clinical benefit in RNA viral infections. Front Nutr. 2020;7:164. Available from: https://doi.org/10.3389/fnut.2020.00164
- Gaudelli NM, Komor AC, Rees HA, Packer MS, Badran AH, Bryson DI, Liu DR. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature. 2017 Nov 23;551(7681):464-471. Available from:https://doi.org/10.1038/nature24644
- Saso L, Orhan HG, Stepanić V. Modulators of oxidative stress: chemical and pharmacological aspects. Antioxidants (Basel). 2020;9(8):657. Available from: https://doi.org/10.3390/antiox9080657
- Svoboda T. 2020 Grothman statement on fenofibrate’s effect on COVID-19 [Internet]. Glenbeulah (WI): Congressman Glenn Grothman; 2020. Available from: https://grothman.house.gov/news/documentsingle.aspx?DocumentID=1669
- Kalousová M. Patobiochemie ve schématech. 1st ed. Praha: Grada; 2006.
- Zheng Y, Zhu D. Molecular hydrogen therapy ameliorates organ damage induced by sepsis. Oxid Med Cell Longev. 2016;2016:5806057. Available from: https://doi.org/10.1155/2016/5806057
- Muhammad Y, Kani YA, Iliya S, Muhammad JB, Binji A, El-Fulaty AA, et al. Deficiency of antioxidants and increased oxidative stress in COVID-19 patients: A cross-sectional comparative study in Jigawa, Northwestern Nigeria. SAGE Open Med. 2021;9:2050312121991246. Available from: https://doi.org/10.1177/2050312121991246
- Slezák J, Kura B, Frimmel K, Zálešák M, Ravingerová T, Viczenczová C, et al. Preventive and therapeutic application of molecular hydrogen in situations with excessive production of free radicals. Physiol Res. 2016;65 Suppl 1:S11–28. Available from: https://doi.org/10.33549/physiolres.933414
- Dasuri K, Zhang L, Keller JN. Oxidative stress, neurodegeneration, and the balance of degradation and protein synthesis. Free Radic Biol Med. 2013;62:170–85. Available from: http://dx.doi.org/10.1016/j.freeradbiomed.2012.09.016
- Ameer K. Avocado as a major dietary source of antioxidants and its preventive role in neurodegenerative diseases. Adv Neurobiol. 2016;12:337–54. Available from: https://doi.org/10.1007/978-3-319-28383-8_18
- Chmátalová Z, Skoumalová A. Oxidative stress in Alzheimer's disease and its consequences. Klin Biochem Metab. 2014;43(4):189–95. Available from: https://casopiskbm.cz/pdfs/kbm/2014/04/04.pdf
- Veech RL. The determination of the redox state and phosphorylation potential in living tissues and relationship to metabolic control of disease phenotype. Biochemistry and Metabolic Biology. 2006;34(3):168–79. Available from: https://doi.org/10.1002/bmb.2006.49403403168
- Gilgun-Sherki Y, Melamed E, Offen D. Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology. 2001;40(8):959–75. Available from: https://doi.org/10.1016/s0028-3908(01)00019-3
- Babbs CF. Free radicals and the etiology of colon cancer. Free Radic Biol Med. 1990;8(2):191–200. Available from: https://doi.org/10.1016/0891-5849(90)90091-v
- Holecek V, Kulich V. [Influence of human erythrocytes on the synthesis of nicotinamide mononucleotide in vitro]. Clin Chim Acta. 1962;7:652–6. Available from: https://doi.org/10.1016/0009-8981(62)90146-8
- Lang CA, Mills BJ, Lang HL, Liu MC, Usui WM, Richie JP Jr, Mastropaolo W, Murrell SA. High blood glutathione levels accompany excellent physical and mental health in women ages 60 to 103 years. J Lab Clin Med. 2002;140(6):413–7. Available from: https://doi.org/10.1067/mlc.2002.129504
- Ortolani O, Conti A, De Gaudio AR, Moraldi E, Novelli GP. [Glutathione and N-acetylcysteine in the prevention of free-radical damage in the initial phase of septic shock. Recenti Prog Med. 2002;93(2):125–9. Available from: https://pubmed.ncbi.nlm.nih.gov/11887346/