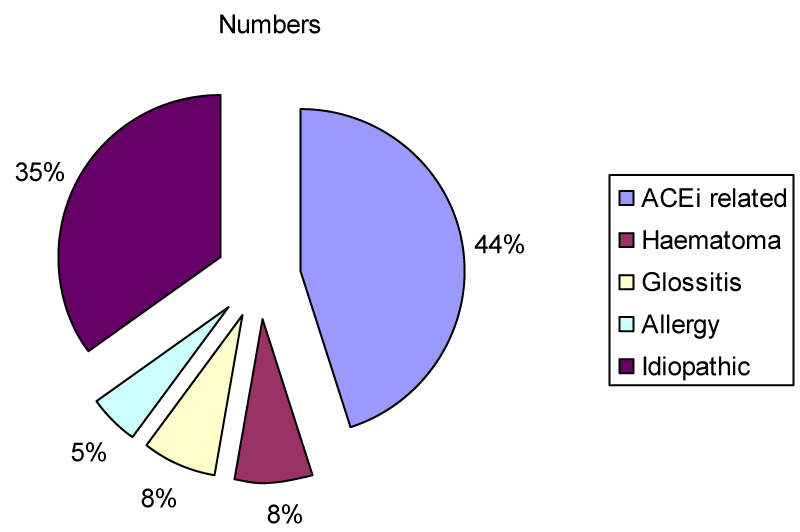More Information
Submitted: 14 June 2020 | Approved: 13 July 2020 | Published: 14 July 2020
How to cite this article: Keh SM, Hasan M, Vallamkondu V, Shakeel M. Management of acute tongue swelling. Heighpubs Otolaryngol Rhinol. 2020; 4: 012-017.
DOI: 10.29328/journal.hor.1001020
Copyright License: © 2020 Keh SM, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Tongue swelling; Acute upper airway obstruction; Angioedema; Haematoma; Anaphylaxis; Allergy
Management of acute tongue swelling
Siew Min Keh1, Mohammad Hasan2, Vamsidhar Vallamkondu3 and Muhammad Shakeel4*
1Consultant Otolaryngologist, Department of Otolaryngology-Head and Neck Surgery, Glasgow Royal Infirmary, Glasgow, UK
2Core Surgical Trainee, Department of General Surgery, Aberdeen Royal infirmary, Aberdeen, UK
3Consultant Otolaryngologist, Department of Otolaryngology-Head and Neck Surgery, Aberdeen Royal infirmary, Aberdeen, UK
4Consultant ENT / Thyroid Surgeon, Department of Otolaryngology-Head and Neck Surgery, Aberdeen Royal infirmary, Aberdeen, UK
*Address for Correspondence: Muhammad Shakeel, Consultant ENT / Thyroid Surgeon, Department of Otolaryngology-Head and Neck Surgery, Aberdeen Royal infirmary, Foresterhill Road, Aberdeen, AB252ZN, UK, Email: [email protected]
Background: Tongue swelling often presents as an acute upper airway obstruction.
Aim: To present a case series of patients presenting with an acute tongue swelling sharing our experience in managing these patients.
Subjects and methods: A retrospective analysis of consecutive patients presenting acutely to the emergency department (ED) at two institutions in Scotland. All patients were evaluated by an otolaryngologist for probable causes of tongue swelling. Data were collected on demographics, co-morbidities, clinical history, examination findings, acute airway management and subsequent care the patients needed.
Results: A total of 32 patients (mean age ± STD, 61.6 ± 18.8; 65% male) were included in the study from two teaching hospitals. The most common presenting symptoms were difficulty in speaking (30/32, 94%) and dysphagia (27/32, 84%). Breathing difficulty was only observed in 8 of 32 patients (25%). Angiotensin converting enzyme (ACE) inhibitor’s induced angioedema was the most common cause (45%) for acute tongue swelling. Three (9.4%) patients required intubation; 2 (6.3%) on initial presentation. Two patients had emergency tracheostomy for breathing difficulties due to supraglottic swelling on flexible pharyngolaryngoscopy.
Conclusion: Acute tongue swelling is a life-threatening condition. The patients on ACE inhibitors would appear to be at higher risk of developing acute tongue swelling. Such patients with potentially compromised airway need to be treated in a facility where emergency intubation and tracheostomy can be performed at a short notice.
Acute tongue swelling can present as an upper airway obstruction. There are a number of pathologies which potentially can cause tongue swelling include angioedema, anaphylaxis, drug rash with eosinophilia and systemic symptom (DRESS) syndrome, local allergic reaction, trauma resulting in tongue haematoma or lingual abscess, viral infections (herpes simplex virus, herpes zoster virus, Coxsackie virus), bacterial infection, foreign body and neoplasia.
Aim
To present a case series of patients presenting with an acute tongue swelling sharing our experience in managing these patients.
A retrospective analysis of patients presenting acutely to the emergency department (ED) with acute tongue swelling was carried out. The relevant patients were identified from our ear, nose and throat (ENT) ward admission books.
The patients identified to have an acute tongue swelling during the study period were included in this study. All other patients presenting with airway issues secondary to causes like supraglottitis or trauma were excluded.
All patients were evaluated by an otolaryngologist for probable causes of tongue swelling. This included a detailed clinical history, known allergies, previous allergic reactions, medication history, family history of hereditary angioedema (HAE) and clinical examination. All patients underwent fiber-optic pharyngolaryngoscopy, which was repeated serially depending on findings. Investigations included full blood count, electrolytes, IgE, complement levels (C3, C4), C1 esterase inhibitor and thyroid function tests if indicated.
Data were collected on demographics, co-morbidities, clinical history, examination findings, acute airway management and subsequent care the patients needed. Microsoft excel was used for data collection and analysis.
A total of 32 patients (mean age ± STD, 61.6 ± 18.8; 65% male) with 40 admissions were included in the study. Each admission is regarded as an individual case for analysis and discussion purposes.
Twenty eight of 40 cases (70%) presented to the emergency department (ED) directly and 11 cases (27.5%) were referred directly to ENT department from their general practitioner (GP), with the remaining 1 patient seen as an outpatient.
Seven of 32 patients (22%) had a known allergy, 10 patients (31.2%) claimed to suffer from a similar tongue swelling in the past and 5 out of 32 patients (15.6%) had more than 1 documented hospital admission with tongue swelling (Table 1).
| Table 1: Patients presenting >1 documented tongue swelling. | |||
| Patient No | Number of presentations / admissions | Probable cause of tongue swelling | Outcome |
| 1 | 2 / 2 | ACE inhibitor | Complete resolution of symptoms with medical treatment |
| 2 | 2 / 2 | Idiopathic | Second admission requiring intubation |
| 3 | 4 / 1 | Idiopathic | Complete resolution of symptoms with medical treatment |
| 4 | 4 / 2 | ACE inhibitor | Complete resolution of symptoms with medical treatment |
| 5 | 5 / 2 | Idiopathic | Complete resolution of symptoms with medical treatment |
| 6 | 5 / 5 | Idiopathic | Complete resolution of symptoms with medical treatment |
The most common presenting symptoms were difficulty in speaking (33/40, 83%) and dysphagia (31/40, 78%). Breathing difficulty was only observed in 8 of 40 patients (20%). ACE inhibitor was the most common cause (45%) of acute tongue swelling followed by idiopathic tongue swelling (35%, Figure 1).
Figure 1: Aetiology of acute tongue swelling; ACEi = ACE inhibitor.
Respiratory rate of patients at presentation ranged from 10 to 28 breaths per minute and there was no correlation between respiratory rate and rate of intubation. However, patients with low oxygen saturation (below 90%) required intubation, as seen in 2 of our patients. Heart rate at presentation ranged from 44 to 128 beats per minute and this had direct correlation with patient’s systolic blood pressure but no influence on the rate of intubation or tracheostomy was noticed.
Seven of 40 cases had raised white cell count and there was no registered electrolyte imbalance in any of the cases on admission. The raised white cell count is thought to be secondary to another co-existing infection. Complements level were all normal apart from in 1 patient who developed angioedema, most likely caused by ACE inhibitor as the patient’s C1 esterase inhibitor level was normal.
All patients received intravenous systemic steroids at initial presentation. Apart from steroids, 22 cases (55%) were treated with nebulized adrenaline. Intramuscular adrenaline was given in 6 cases (15%) and 5 of these 6 cases were also given nebulized adrenaline. Majority of patients received antihistamines as part of their treatment (78%). Antibiotic was prescribed in 8 of 40 cases (20%) managed.
Despite initial treatment, 3 (9.4%) patients required intubation; 2 (6.3%) on initial presentation due to low oxygen saturation (< 90%). One of these was readmitted twice and, on both admissions, the patient required intubation due to severity of tongue swelling. Four (12.5%) patients required tracheostomy. Two patients had emergency tracheostomy for breathing difficulties due to supraglottic swelling on flexible pharyngolaryngoscopy. One patient had to have semi-elective tracheostomy tube insertion in order to wean patient off the ventilator. One patient who was initially intubated progressed to surgical tracheostomy due to pulmonary sepsis.
All of our patients made a successful recovery.
Tongue swelling often presents acutely with either with swallowing difficulty and/or in conjunction with dysarthria with or without breathing difficulties [1]. In our experience the dysarthria was one of the most common presenting symptoms.
Overall, there are few studies examining the epidemiology of ED attendance for tongue swelling and angioedema. The reason for the latter is lack of understanding and consensus amongst clinicians on the case definition and specifically the distinction between different groups of allergic reaction [1].
A multicentre retrospective study conducted at 5 academic EDs, revealed that 30% of adult ED patients with angioedema had ACE inhibitor angioedema, with 18% of these being admitted for observation at a short stay unit, 12% were admitted to inpatient units and 11% admitted to ITU [2]. Bluestein and colleagues [3] found that 30% of angioedema cases were related to ACE inhibitor. In their series, there was a lower admission rate of 14%. ACE inhibitor should be suspected as a cause of tongue swelling in patients with no other obvious cause found in clinical history preceding to the event, though the incidence is only approximately 0.1% to 0.7% and usually within the first 30 days of commencement of medication. The oedema is mainly confined to the lip and tongue [3]. African origin and patients on immunosuppressants are at significant enhanced risk in developing this condition and this can occur at anytime, even years after starting the ACE inhibitor. Other non-histamine-mediated drug reaction includes nonsteroidal anti-inflammatory drugs (NSAID) due to accumulation of leukotriene mediators from inhibition of cyclooxygenase [1].
In our case series, majority of patients presented with angioedema were related to the use of ACE inhibitors. Over the last 50 years, there is an increase in use of ACE inhibitor to regulate blood pressure and cardiovascular system. Angioedema has been documented as an adverse effect of ACE inhibitor at least in the last 4 decades [4-7] with fatalities reported resulting from upper airway obstruction [8] due to increased levels of bradykinin [9,10]. However, a 2012 meta-analysis of randomized trials of angioedema as an adverse event of ACE inhibitor concluded an overall incidence of angioedema with ACE inhibitor was 0.11% which was not significantly different from placebo (0.07%) [11]. They reported a higher incidence of angioedema in angiotensin receptor blocker treated heart failure trials compared to coronary artery disease without heart failure and hypertension [11].
A retrospective case study of 183 patients with ACE inhibitor induced angioedema found the intubation rate to be 9.5% which is comparative to our study (9.4%). This study found that intubation was more likely in patients presenting within 6 hours of symptoms. It also reported that patients with anterior tongue swelling, vocal changes, drooling and dyspnea were more likely to require intubation [12].
Acquired angioedema related to the use of an ACE inhibitor can present even after a single dose. A 60-year-old woman was reported to have presented with swollen tongue and face with impending airway obstruction [13] having taken a single dose the day before. No other signs of anaphylaxis were present. Adrenaline and steroids were ineffective and she eventually needed a tracheostomy with intensive care admission. She went on to make a full recovery.
An interesting case report in 2018 mentioned an 86-year-old woman with a background of angioedema, who was found dead at home. Autopsy revealed a grossly edematous tongue with associated oedema of the tonsillar fossae, epiglottis and glottic inlet causing critical obstruction [14]. Lingual angioedema has also been reported as a side effect after alteplase treatment in ischaemic stroke [15].
Initial patient evaluation should comprise of detailed history taking and physical examination. A case report in 2017 reported a 22-year-old lady who presented with lingual haematoma secondary to self-biting following an acute dystonic reaction. This swelling was initially thought to be angioedema, but a thorough history pointed to her previous psychiatric history and use of anti-psychotic medications [16]. This reiterates the importance of a thorough history.
Basic laboratory investigations may be useful in ruling out severe systemic disease. Investigations should be directed towards identifying the underlying cause for the tongue swelling in order for treatment to be directed towards correcting this and preventing secondary insult. In the presence of urticaria with tongue swelling or if an allergic reaction is suspected, specific provocation for example with pseudo-allergen rich diet, differential blood count and ESR may be helpful for identification of underlying causes. Extended diagnostic testing is aimed at patients who suffer from recurrent severe symptoms. The tests are also undertaken to rule out possible differential diagnoses if indicated.
A normal complement study, especially during an acute attack, essentially excludes C1 inhibitor deficiency. Idiopathic angioedema occurs when mast cells degranulate spontaneously rather than in response to specific allergen. This is may be supported by clear evidence of dermatographism, along with a history of pruritus and urticaria after physical stimuli such as heat or pressure which provide evidence of mast cell instability. In most individuals, no cause is found for idiopathic angioedema, but on occasion this can be associated with abnormalities in thyroid function and anaemia. All patients diagnosed with idiopathic tongue swelling in this study have normal thyroid function test, haemoglobin and complement factors (C3 and C4).
The initial management should follow the advanced life support (ALS) guidelines. Maintenance of the airway is a priority and all patients should be nursed ideally in a high dependency unit or in an ENT ward under close observation, near a nursing station with frequent vital signs assessment. Although majority of patients may display normal haemodynamic parameters, a small number of patients may exhibit tachycardia, hypotension and respiratory failure as a result of fluid shifts between various body compartments due to vascular permeability and airway oedema leading to asphyxiation.
In the presence of impending airway concerns, reassessment should be performed immediately to secure the airway by intubation by an experienced anaesthetist. Further diagnostic evaluation with flexible pharyngolaryngoscopy would help to determine airway patency. To date, a validated clinical decision algorithm to identify which patients require pharyngolaryngoscopy is still lacking. The Ishoo classification for monitoring severity of upper airway may be used to assess risk and admission though these criteria have yet to be validated [17].
In this case series, the authors observe that the location of oedema is an important factor leading to an early intubation to protect the airway. In our experience emergency intubation is more likely to be needed when oedema involves the pharynx and larynx compared to lip and face oedema. Patients who required intubation after disease progression between serial evaluations, were more likely to have oedema involving the upper aerodigestive tract. Patients’ age and co-morbidities did not influence the rate of intubation.
Subsequently, steps should be taken to stabilize patient’s condition and prevent secondary insult. The role of corticosteroids has been well documented in the medical literature. Steroids act by inhibiting phospholipase A2 leading to inhibition of arachidonic acid metabolism and platelet activation factor required for clotting. Its use is well documented in the treatment of acute infective or inflammatory condition in the head and neck, e.g. acute epiglottitis or supraglottitis, severe tonsillitis or glandular fever without any complications. However, the use of corticosteroid in an acute setting may mask an ongoing infection or lead to an increase in infective process. Therefore, its use may be controversial.
In angioedema, corticosteroids, antihistamines and adrenaline are mainstay of treatment agents for this condition. A prospective cohort observational study conducted on 40 patients found that emergency treatment with corticosteroids and antihistamines in patients with ACE inhibitor induced angioedema, showed effective resolution and no deaths related to this condition were reported [18]. However, these are ineffective in treating bradykinin-mediated angioedema. This includes hereditary and ACE inhibitor.
The European Academy of Allergy and Clinical Immunology has issued a consensus report from the Hereditary Angioedema (HAE) Internal Working Group on classification of angioedema without wheals into hereditary and acquired; diagnosis and evidence-based recommendation for the treatment for angioedema [19]. This was then revised in 2017 [20] and reclassified into HAE with deficient C1-inhibitor (type 1), HAE with dysfunctional C1-inhibitor (type 2), HAE with mutation in the angiopoietin-1 gene (HAE-ANGPT1), HAE with mutation in the plasminogen gene (HAE-PLG) and those with unknown mutation (HAE-UNK) to further aid with diagnosis and appropriate treatment decisions.
Bradykinin-mediated angioedema responds poorly to epinephrine and tends to be more severe, longer lasting and much more likely to involve concurrent abdominal symptoms than histamine-mediated angioedema [21]. The use of adrenaline in patients who are on ACE inhibitors to treat hypertension, especially elderly patients with cardiovascular disease, may result in elevated cardiovascular risk. Sedating antihistamines must be avoided in all patients with potential upper airway compromise. Bradykinin antagonists have become an emerging therapy for these patients [21]; though their role in angioedema related to use of angiotensin receptor blocker has not been fully established. A 75-year-old female was reported to have presented with lingual angioedema secondary to ACE inhibitor use. She was unresponsive to antihistamines, steroids and adrenaline but was successfully treated with one dose of icatibant (bradykinin receptor antagonist) and hence avoided intubation or mechanical ventilation [22].
A randomised control trial (RCT) of 27 patients with ACE inhibitor induced angioedema showed the time to complete resolution of oedema was significantly shorter with icatibant than with treatment with steroids and antihistamines [23]. However, this study is in contrast to other clinical trials. An RCT conducted on 33 patients with ACE inhibitor associated angioedema to establish the efficacy of icatibant (bradykinin receptor antagonist), failed to establish any clinical efficacy over the placebo [24]. The same results were obtained in another RCT involving 121 patients [25].
In contrast to bradykinin-mediated angioedema, allergic and idiopathic angioedema with or without concomitant urticaria or evidence of anaphylaxis are mast cell-mediated by histamine release. Therefore, these conditions will respond to antihistamine, epinephrine or corticosteroids. A baseline diagnostic laboratory test should include C1-INH protein level and function, and C4 profile level.
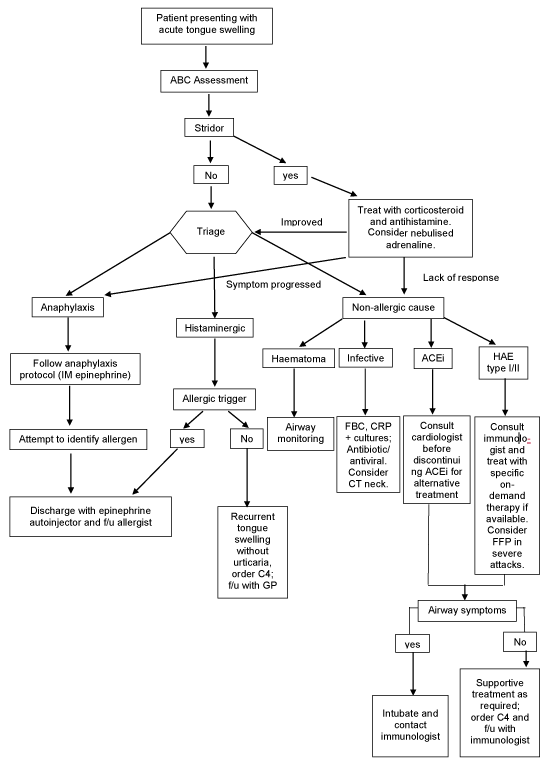
We have created a simple flow chart to manage acute tongue swelling (Figure 2). Before discharging patient home, a well-formulated plan with follow-up to appropriate specialist must be in place. Patient’s diagnosis should be established and documented clearly in the discharge letter. In cases of allergy reaction, patient and GP should be informed and allergen avoidance is advised.
Figure 2: Flow chart of management of acute tongue swelling. History taking and examination to establish possible cause of tongueswelling and management; ABC – airway, breathing, circulation; IM - intramuscular; ACEi - ACE inhibitor; HAE – hereditary angioedema; f/u - follow-up; GP - general practitioner, FFP – Fresh frozen plasma.
If a patient has a history of severe allergic reaction with recurring episodes, patient may be provided with an epinephrine on hand to be used in similar situation and refer to an immunologist. The patients with ACE inhibitor induced tongue swelling should have their medication discontinued following consultation with the cardiologist or medical team and consideration should be given to an alternative antihypertensive medication such as calcium channel blocker. The patient’s GP must be contacted to maintain chain of communication.
If the patient’s tongue swelling was unresponsive to antihistamine and corticosteroids, and there is a family history of angioedema attacks, it is important to arrange follow-up with an immunologist for hereditary angioedema (HAE) assessment. The laboratory tests, particularly C4 and tryptase levels can be taken during an acute attack. These patients should be educated on HAE triggers, for example enhanced risk with certain medications (oestrogen and ACE inhibitor) and precautions to be taken during dental work and surgery. Patients known to suffer from HAE are more likely to have an emergency treatment plan, home treatment options and received training in self-administration of rescue medication to reduce hospital readmission. The patients with upper airway symptoms and signs should be encouraged to self-treat without delay.
Acute tongue swelling is a life-threatening condition. The patients on ACE inhibitors would appear to be at higher risk of developing acute tongue swelling. Such patients with potentially compromised airway need to be treated in a facility where emergency intubation and tracheostomy can be performed at a short notice.
- Moellman JJ, Bernstein JA, Lindsell C, Banerji A, Busse PJ, et al. A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med. 2014; 21: 469-484. PubMed: https://pubmed.ncbi.nlm.nih.gov/24730413
- Banerji A, Clark S, Blanda M, LoVecchio F, Snyder B, et al. Multicenter study of patients with angiotensin-converting enzyme inhibitor-induced angioedema who present to the emergency department. Ann Allergy Asthma Immunol. 2008; 100: 327-332. PubMed: https://pubmed.ncbi.nlm.nih.gov/18450117/
- Bluestein HM, Hoover TA, Banerji AS, Camargo CA Jr, Reshef A,et al. Angiotensin-converting enzyme inhibitor-induced angioedema in a community hospital emergency department. Ann Allergy Asthma Immunol. 2009; 103: 502-507. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20084844
- Gabb GM, Ryan P, Wing LM, Hutchinson KA. Epidemiological study of angioedema and ACE inhibitors. Aust N Z J Med. 1996; 26: 777-782. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9028507
- Wood SM, Mann RD, Rawlins MD. Angio-oedema and urticaria associated with angiotensin converting enzyme inhibitors. Br Med J (Clin Res Ed). 1987; 294: 91-92. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1245098/
- Lunde H, Hedner T, Samuelsson O, Lötvall J, Andrén L, et al. Dyspnoea, asthma, and bronchospasm in relation to treatment with angiotensin converting enzyme inhibitors. BMJ. 1994; 308: 18-21. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2539116/
- Weiner JM. Failure to recognise the association of life-threatening angio-oedema and angiotensin-converting enzyme inhibitor therapy. Aust N Z J Med. 1995; 25: 241-242. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7487694
- Andrew N, Gabb G, Del Fante M. Aust Fam Physician. ACEI associated angioedema - a case study and review. 2011; 40: 985-988. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22146327
- Anderson MW, deShazo RD. Studies of the mechanism of angiotensin-converting enzyme (ACE) inhibitor-associated angioedema: the effect of an ACE inhibitor on cutaneous responses to bradykinin, codeine, and histamine. J Allergy Clin Immunol. 1990; 85: 856-858. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2185292
- Ferner RE, Simpson JM, Rawlins MD. Effects of intradermal bradykinin after inhibition of angiotensin converting enzyme. Br Med J (Clin Res Ed). 1987; 294: 1119-1120. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/3034372
- Makani H, Messerli FH, Romero J, Wever-Pinzon O, Korniyenko A, et al. Meta-analysis of randomized trials of angioedema as an adverse event of renin-angiotensin system inhibitors. Am J Cardiol. 2012; 110: 383-391. PubMed: https://pubmed.ncbi.nlm.nih.gov/22521308/
- Mudd PA, Hooker EA, Stolz U, Hart KW, Bernstein JA, et al. Moellman. Emergency department evaluation of patients with angiotensin converting enzyme inhibitor associated angioedema. 2020; PubMed:
- Krogh Nielsen T, Bygum A, Rye Rasmussen E. Life-threatening angio-oedema after the first dose of an ACE inhibitor-not an anaphylactic reaction. BMJ Case Rep. 2016; 2016: bcr2016214364. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27229746
- Gilbert JD, Byard RW. Lethal manifestations of angioedema. Forensic Sci Med Pathol. 2019; 15; 494–497. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30390279
- Bozkurt S, Arslan ED, Köse A, Ayrık C, Yılmaz A, et al. Lingual angioedema after alteplase treatment in a patient with acute ischemic stroke. World J Emerg Med. 2015; 6: 74‐76. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4369536/
- Sezer Ö, Aydin AA, Bilge S, Arslan F, Arslan H. Acute dystonic reaction leading to lingual hematoma mimicking angioedema. Indian J Pharmacol. 2017; 49: 325‐327. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29326495
- Ishoo E, Shah UK, Grillone GA, Stram JR, Fuleihan NS. Predicting airway risk in angioedema: staging system based on presentation. Otolaryngol Head Neck Surg. 1999; 121: 263-268. PubMed: https://pubmed.ncbi.nlm.nih.gov/10471868/
- Al‐Khudari S, Loochtan MJ, Peterson E, Yaremchuk KL. Management of angiotensin‐converting enzyme inhibitor–induced angioedema. The Laryngoscope. 2011 121: 2327-2334. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4241279/
- Cicardi M, Aberer W, Banerji A, Bas M, et al. (European Academy of Allergy and Clinical Immunology). Classification, diagnosis, and approach to treatment for angioedema: consensus report from the Hereditary Angioedema International Working Group. Allergy. 2014; 69: 602-616. PubMed: https://pubmed.ncbi.nlm.nih.gov/24673465
- Maurer M, Magerl M, Ansotegui I, Aygören-Pürsün E, Betschel S, et al. The international WAO/EAACI guidelines for the management of hereditary angioedema – the 2017 revision and update. Allergy. 2018; 73: 73(8):1575-1596. PubMed: https://pubmed.ncbi.nlm.nih.gov/29318628/
- Cicardi M, Banerji A, Bracho F, Malbrán A, Rosenkranz B, et al. Icatibant, a new bradykinin-receptor antagonist, in hereditary angioedema. N Engl J Med. 2010; 363: 532-541. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4662377/
- Ostenfeld S, Bygum A, Rasmussen ER. Life-threatening ACE inhibitor-induced angio-oedema successfully treated with icatibant: a bradykinin receptor antagonist. BMJ Case Rep. 2015; 2015: bcr2015212891. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26498671
- Baş M, Greve J, Stelter K, et al. A randomized trial of icatibant in ACE-inhibitor-induced angioedema. N Engl J Med. 2015; 372: 418‐425. PubMed: https://pubmed.ncbi.nlm.nih.gov/25629740/
- Straka BT, Ramirez CE, Byrd JB, et al. Effect of bradykinin receptor antagonism on ACE inhibitor-associated angioedema. J Allergy Clin Immunol. 2017; 140: 242‐248.e2 PubMed: https://pubmed.ncbi.nlm.nih.gov/27913306
- Sinert R, Levy P, Bernstein JA, et al. Randomized Trial of Icatibant for Angiotensin-Converting Enzyme Inhibitor-Induced Upper Airway Angioedema. J Allergy Clin Immunol Pract. 2017; 5:1402‐1409.e3. PubMed: https://pubmed.ncbi.nlm.nih.gov/28552382/