Case Report
CT signs of pressure induced expansion of paranasal sinus structures

Peter AR Clement* and Stijn Halewyck
Department of Otolaryngology, Head and Neck Surgery, Universitair Ziekenhuis-Free University Brussels (UZ-VUB), Belgium
*Address for Correspondence: Prof. Peter Clement AR, Department of Otolaryngology, Head and Neck Surgery, Laarbeeklaan 101, B-1090 Brussels, Belgium, Tel: 32-2-477 68 88; Email: [email protected]
Dates: Submitted: 11 September 2017; Approved: 25 September 2017; Published: 26 September 2017
How to cite this article: Clement PAR, Halewyck S. CT signs of pressure induced expansion of paranasal sinus structures. Heighpubs Otolaryngol and Rhinol. 2017; 1: 077-087. DOI: 10.29328/journal.hor.1001013
Copyright License: © 2017 Clement PAR, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Hyper inflated sinus structures; Nose blowing; Chronic rhino sinusitis
Summary
Several articles have been written about hyper inflated sinus structures. Never before, however, a complete overview of all possible pressure induced variations of sinus anatomy have been published. The aim of this study was to make an inventory of the most common CT signs of hyper inflated paranasal sinus structures. During a period of 2 years all CT-scans of the paranasal sinuses made in an ENT-department were studied and the most typical shapes of hyper inflated sinus structures were recorded.
The authors documented 9 different anomalies of the anterior paranasal sinus complex (frontal sinus, frontal and supra-orbital recess and anterior ethmoid), 8 of the ethmoid and 1 of the sphenoidal sinus. These hyper inflated paranasal sinus structures can only be generated by high positive intranasal pressures. The nose blowing manoeuvre is the only manoeuvre that generates extremely high pressures and as such it might be the driving force in the generation of these hyper inflated paranasal structures and consequently play a role in the pathophysiology of chronic sinusitis.
Pneumatisation of the sinuses starts at birth and is a lifelong process. Sometimes, however, pneumatisation can be extreme and will result in facial deformities. Pneumosinus dilatans, is such a condition, characterized by an abnormal dilatation of a paranasal sinus cavity, containing air only. Most reports describe pneumosinus dilatans of the frontal sinus, but also other sinuses can show this phenomenon: maxillary sinus and in one case a unilateral pneumosinus dilatans of nearly all sinuses (maxillary, ethmoid, and sphenoid sinus) was described.
Recently Kalavagunta et al., described a less dramatic expansion of the maxillary sinus and named it “Extensive Maxillary Sinus Pneumatisation” (EMSP). They were surprised to see that EMSP has received little attention in the literature. Neuner et al., described 9 different atypical pneumatisation abnormalities of the paranasal sinus anatomy.
Most of deformities of the sinus pneumatisation are growth deformities of the thick bones that make up the frame of the sinuses. Only a few articles, deal with specific deformities of thinner bone structures such as “wavy orbital floor” and “frontal cells”. Never before, however, an article was published that studied all possible deformities due to increased pressures and tried to make a classification. So the aim of this study was to make an inventory of the most obvious pressures related deformities that can be seen on CT-scans of patients with rhinosinusitis.
Methodology
The investigation consisted of a careful prospective evaluation of all the CT-scans made during a 2 year period in a university outpatient ENT clinic (2002-2003) with the aim to describe all possible signs of bony deformities of the paranasal sinuses that eventually could be attributed to high intranasal pressures. Because the ethmoid is the only bone in the human body that can produce paper thin bony lamellae and as a consequence the bone that most often will show deformities induced by increased pressures, the authors propose a classification that deals with deformities of the paranasal sinuses anterior to the ethmoid, the etmoid and posterior to the ethmoid.
Results
The abnormalities of bony paranasal sinus structures on the CT-scans that could be attributed to increased pressures, observed by the author, can be divided in 3 subgroups: abnormalities of
A. the anterior complex
B. the ethmoid
C. the sphenoid
A. anterior complex (frontal sinus, frontal recess, supra-orbital recess and anterior ethmoid)-coronal scans
1. Presence of bony cells invading the frontal sinus (Figures 2,3A-3C,6A,6B,13A,13B)
2. Hyper pneumatisation of the frontal sinus (Figures 3A,3B,4A,4B)
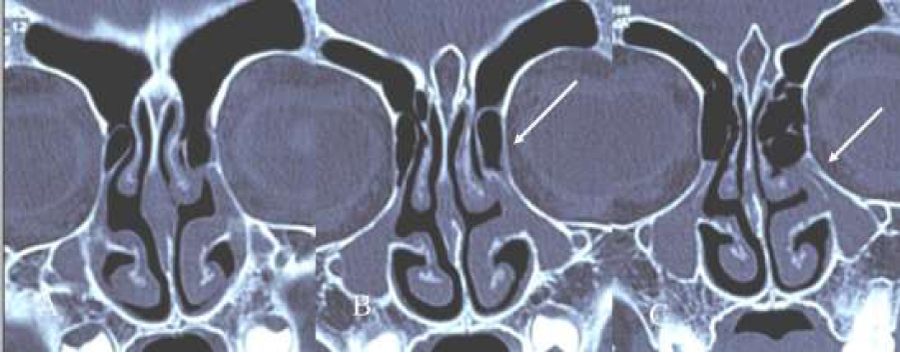
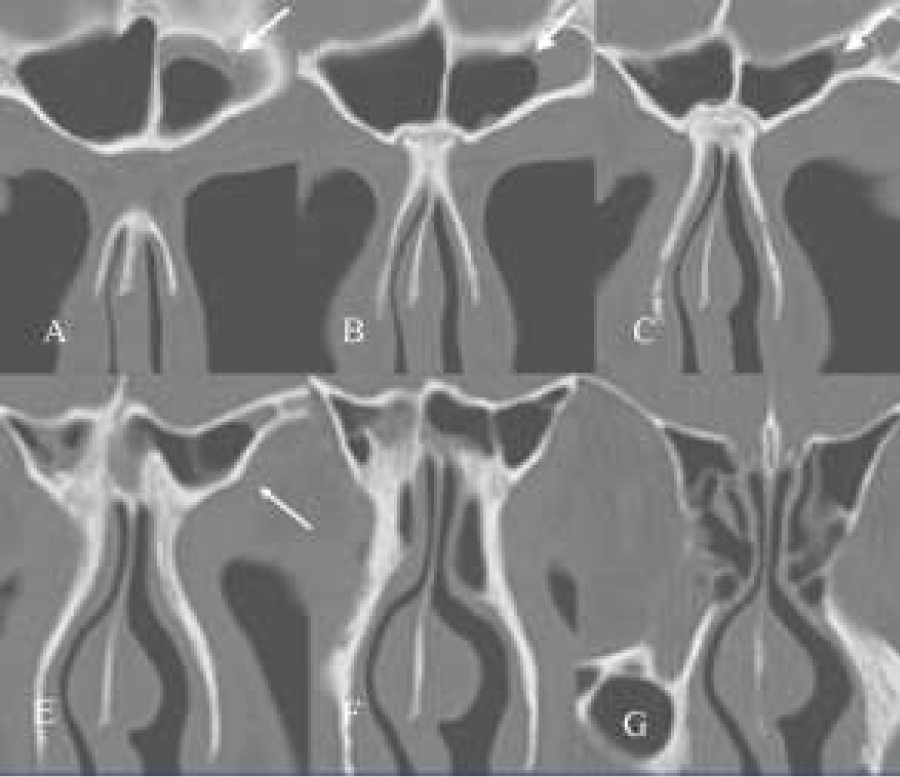
3. Bossing of anterior tabula of frontal sinus and the infundibulum of the frontal sinus (Figure 1)
4. Bossing of orbital rim at the level of the infundibulum of the frontal sinus (Figures 3B,6B,13E)
5. Pneumatisation of the nasal bones, septum and crista galli (Figures 4A-4C,8A,10A)
6. Pneumatisation of the agger nasi (agger nasi cell) (Figures 3A,5A,9A,9B,10B)
7. Rounding of supra-orbital recess (Figures 3C,8A,8B,11A,11B,12A-12C,13C)-parasagital scan
8. Flattening of the anterior skull base (Figure 3D)
9. Lacrimal and frontal recess cells showing “bunch of grapes” aspect (Figure 3D) invading the frontal sinus
B. Ethmoid
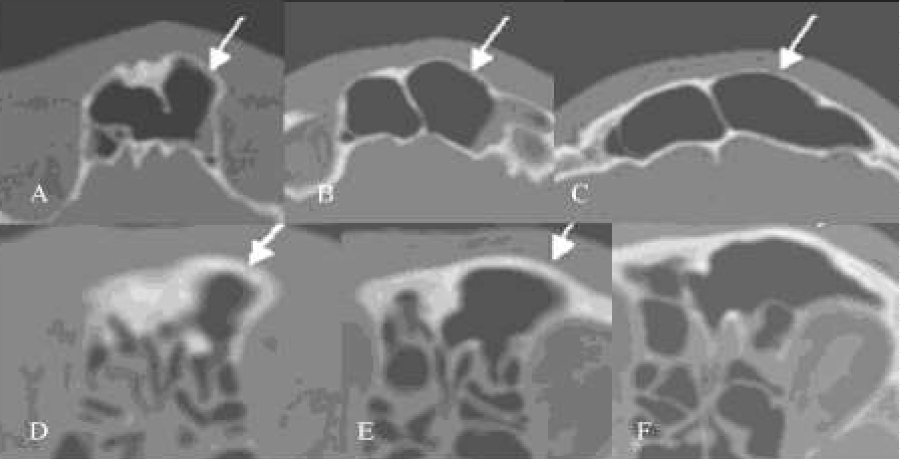
Figure 1: (A-F) Axial CT-scans. Two cases of patients showing bossing of the anterior wall (tabula externa) of the frontal sinus and of the infundibulum of the frontal sinus.
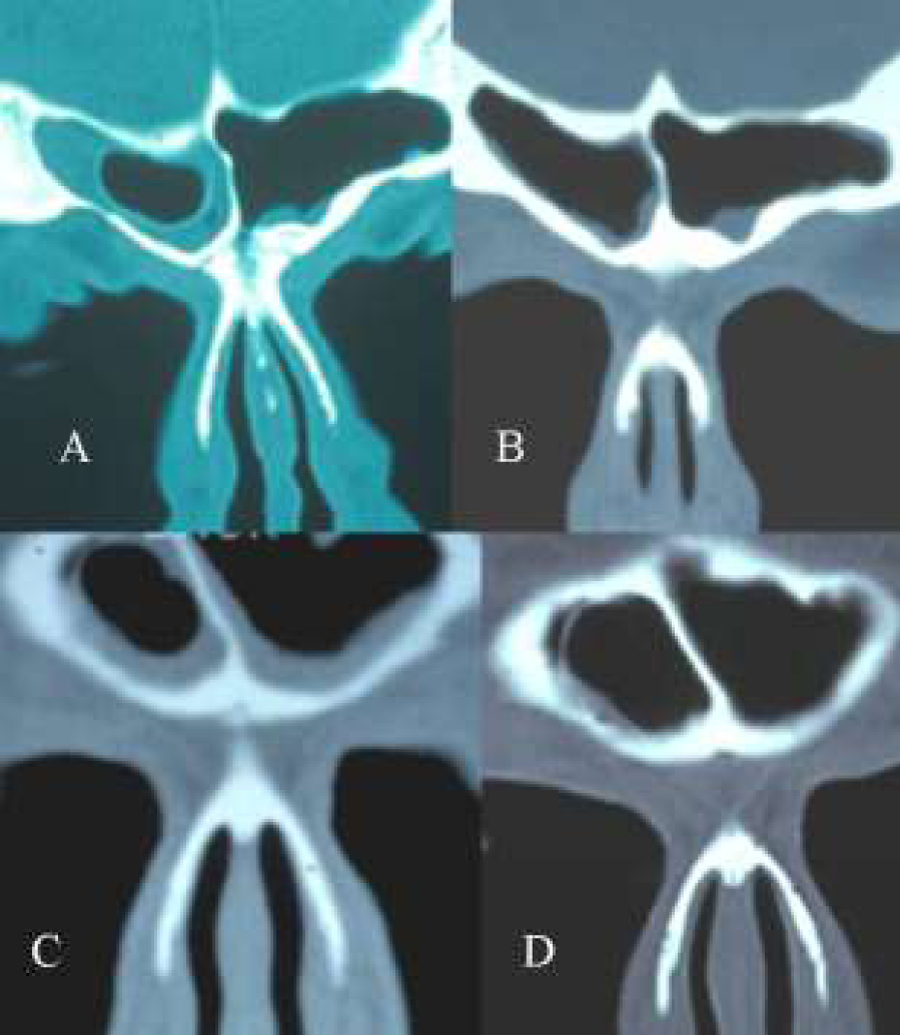
Figure 2: (A-D) Coronal CT-scans. A+B: pre-and postoperative CT-scan of a frontal cell invading the frontal sinus. The interior of the cell is free of disease. After removal of the cell the mucosa of the frontal sinus becomes normal. C+D: Two CT-scans at the same level with a 2 year difference in a patient with a frontal cell in the frontal sinus. Note that the cell has increased in size (D).
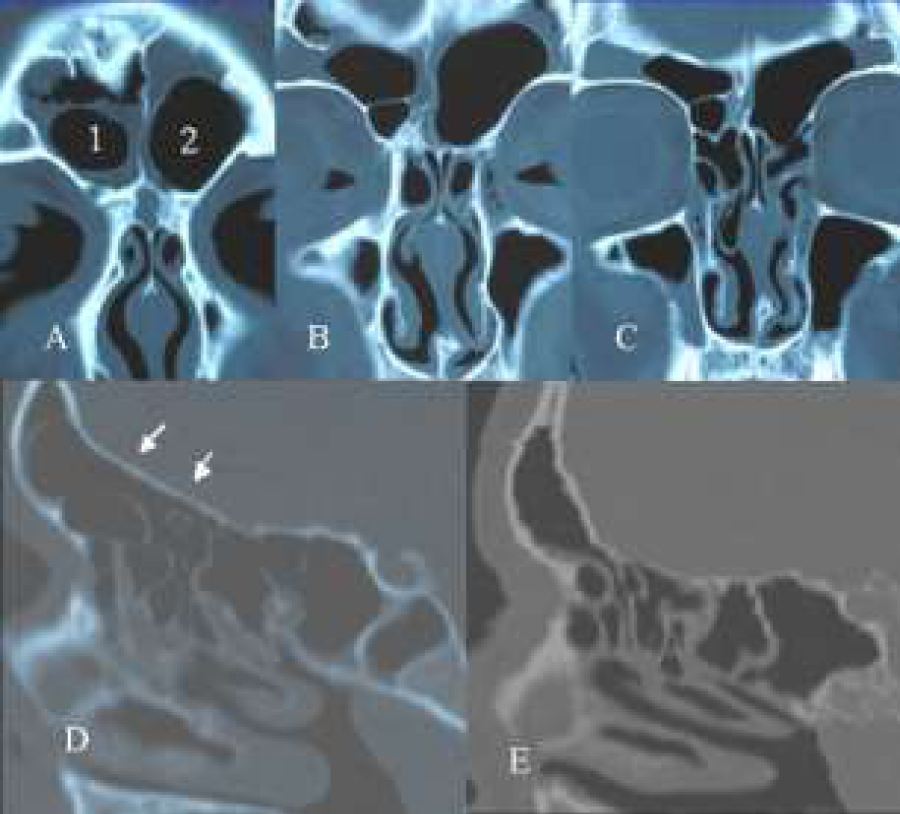
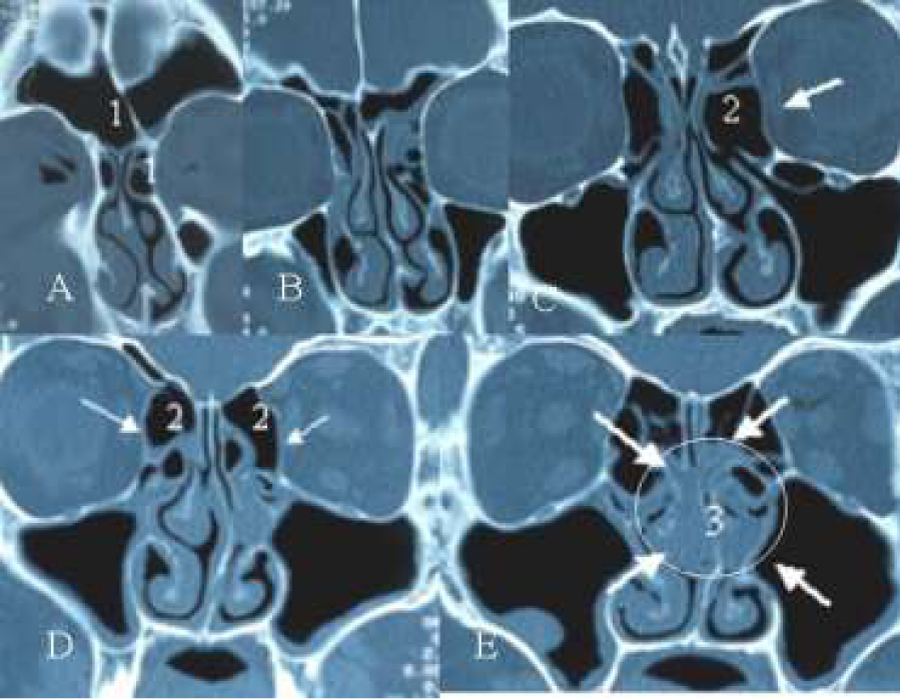
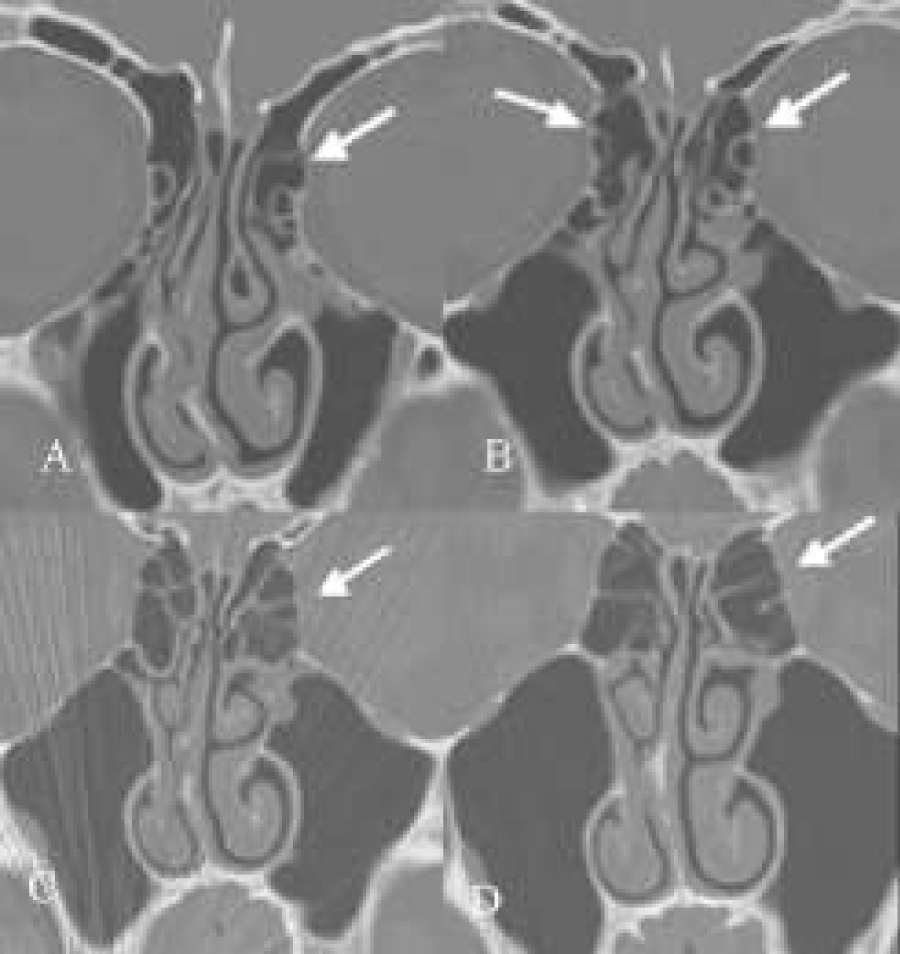
Figure 3: (A-E) A+B+C: Coronal CT-scans. Two frontal cells, right one (1) filling the frontal sinus for 30 % and the left one (2) for more than 50 % (A). Interior of the cells shows no pathology. The frontal sinus on both sides are opaque. In A there are also two agger nasi cells on both sides. Also in B there is a second cell filling on the right side the infundibulum of the frontal sinus, showing some bossing of the orbital rim. C shows a clear-cut hyperinflated anterior frontal complex and supra-orbital recess. On the left side the ostiomeatal complex shows signs of mucosal swelling. Note the presence of a concha media bullosa on the right side. There exists a septal deviation towards the left side (resulting in a paradoxical bending of the inferior turbinate) close to the floor. D. Parasagital CT-scans of the same patient (left side) showing flattening of the skull base due to hyperpneumatisation of the frontal recess and frontal sinus by hyperinflated cells (aspect of a “bunch of grapes”). Compare this configuration of these cells and flattening of the skull base with a normal case (E).
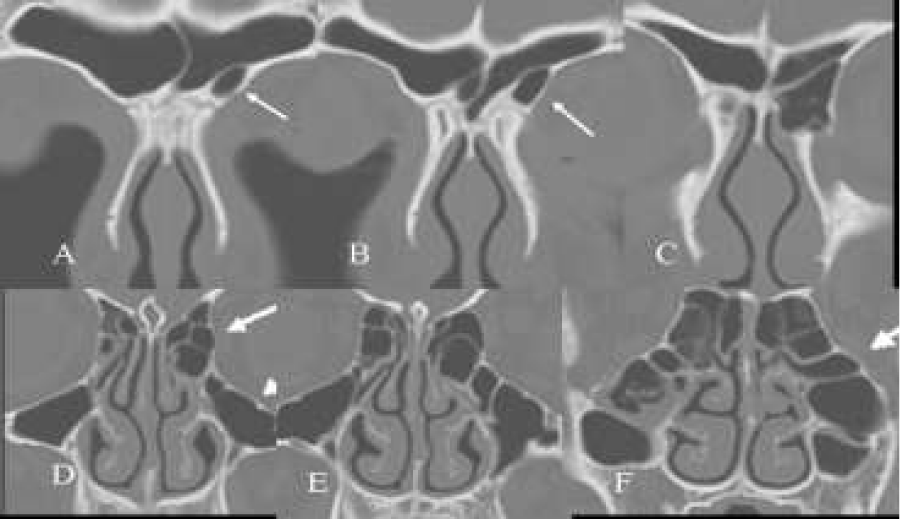
Figure 4: (A-C) Coronal CT-scan showing hyperpneumatised left frontal sinus with pneumatisation of the nasal bones (A) and of the septum (B and C).
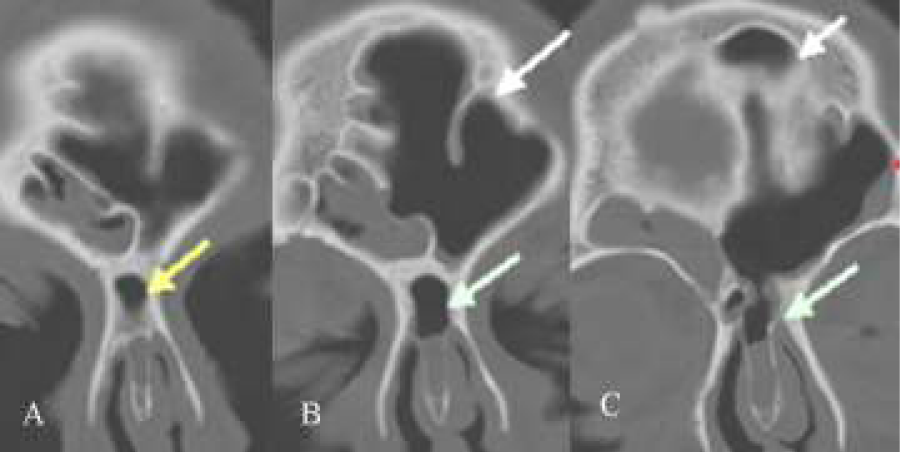
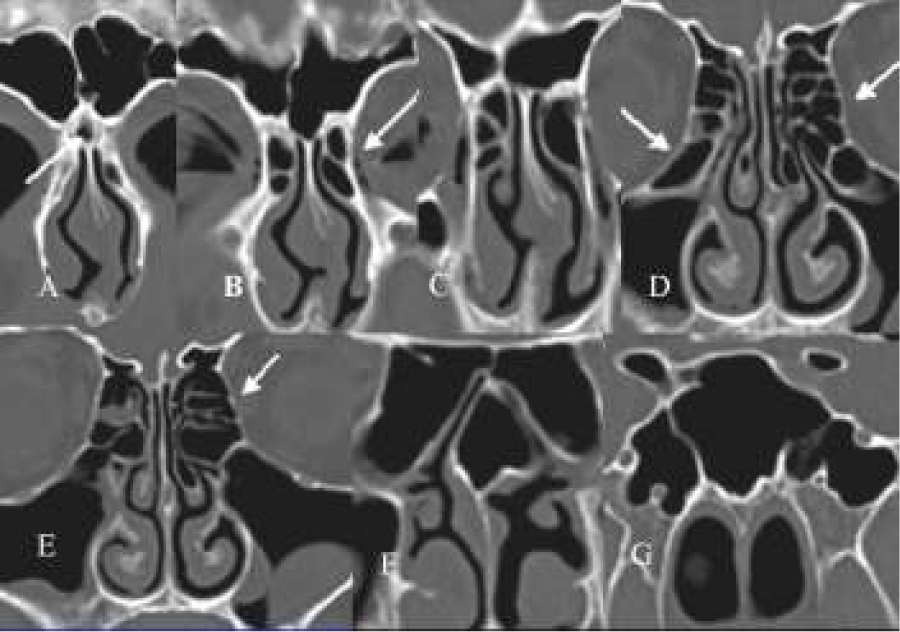
Figure 5: (A-E) Axial CT-scan showing presence of bilateral agger nasi cell (A), hyperinflated bulla ethmoidalis (C2) ballooning of the lamina orbitalis (C and D) and presence of an infra-orbital cell at the left side (C). Note also the diseased mucosa of both ethmoidal infundibulae and around both bullae leading to a beginning of a “black halo” aspect of the disease (E3) on the CT-scan.
Figure 6: (A-F) Coronal CT-scan. Note the presence of a frontal recess cell invading the infundibulum of the frontal sinus leading to bossing of the orbital rim on the left side (A,B), ballooning of the lamina orbitalis left side (D) and waving of the floor of the orbit (left side) (D,E,F). Note also the presence of bilaterally infra-orbital cells, left side (E,F), right side (F). On the right side there are already signs of mucosal disease.
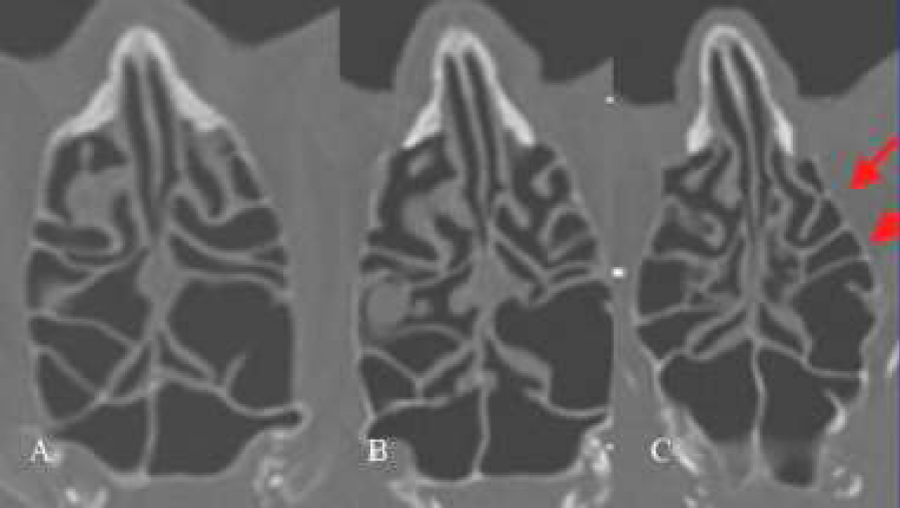
Figure 7: (A-C) Same patient as in Fig. 3 (D,E,F). Axial scans showing ballooning of the complete ethmoid resulting in an “egg-shaped” configuration.
Figure 8: (A-F) Coronal CT-scan. Pneumatisation of the nasal bones (A), rounding of the supra-orbital recess (A,B), signs of ballooning and “cobble stone” aspect of the lamina orbitalis (D,E,F) pointing at a hyperinflated ethmoid (“ethmoidal emphysema”) with signs of mucosal disease mainly around ostiomeatal complex (black halo” D,E) extending in both maxillary sinuses and right anterior ethmoid (C).
- Coronal scan
1. Ballooning (Figures 5C,5D,6D,8D,8E,8F,9A-9D,10D,10E,12C) of lamina orbitalis, and “cobble stone” (Figures 8E,8F,9B-9D,10D,10E) aspect of the lamina orbital./p>
2. Hyper inflated ethmoid (ethmoidal emphysema or nearly empty ehmoid) (Figures 8D-8F,9B-9D,10D,10E,11D-11F,12C): large air cells, signs of ballooning and mostly presence of only a few very thin atrophic bony lamellae
3. Infra-orbital cells (“Haller” cells) (Figures 5C,6E,6F,10D)
4. Pneumatisation of uncinate process (Figures 12A, 12B)
5. Hyper inflated bulla (Figures 5D,5C,9B,9C,11D,11E), concha bullosa (Figures 3C,9A,10D,10E
6. Waving of the orbital floor (Figures 6D-6F,11E,11F)
7. Extensive maxillary sinus pneumatisation (EMSP) (Figures 11D-11F)-axial scan
8. Egg shape of ethmoidal complex (Figure 7)
C. Sphenoid
Hyperpneumatisation of sphenoid: extensive pneumatisation (retro-sellar extension of sphenoid and pneumatisation into the pterygoid process) (Figures 10F,10G,11G,11H).
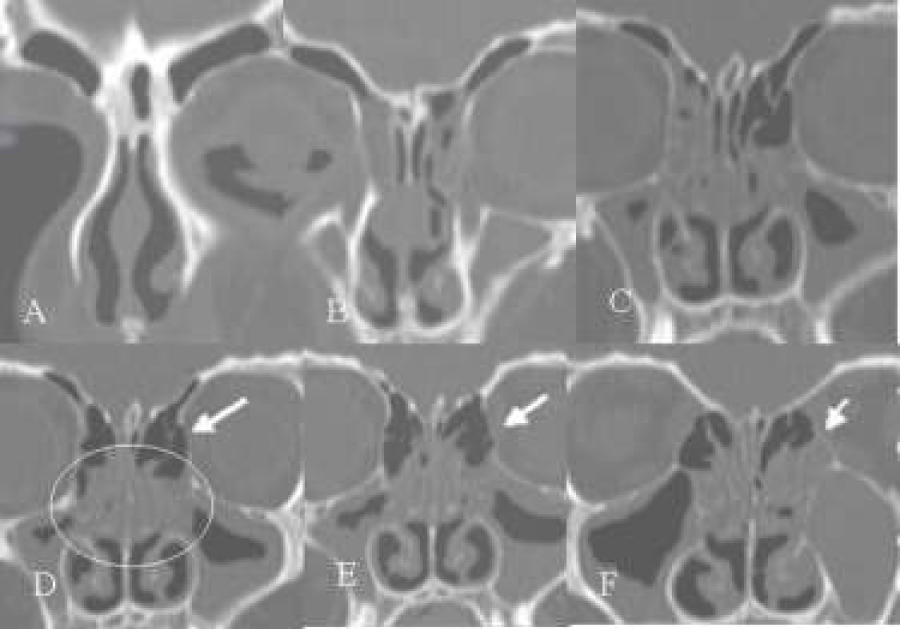
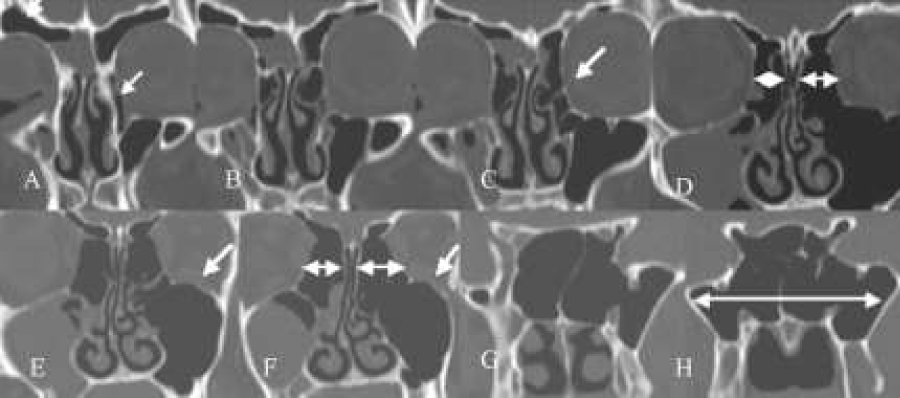
Figure 9: (A-D) Coronal CT-scans. Some rounding of supra-orbital recess (A), hyperinflated ethmoid (with ballooning of both lamina orbitalis, B,C) with clear-cut “cobble-stone”aspect of this orbital lamina on the left side (B,C), hyperinflated bullae bilaterally (B,C) septal deviation to the right and beginning disease of the mucosa in and around in the infundibulum ethmoidale of the left side (A,B,C), and a left concha media bullosa (A).
Figure 10: (A-G) Coronal scans. Pneumatisation of the nasal bones (A), presence of bilateral agger nasi (B,C), hyperinflated ethmoid especially on the left (“ballooning” (D,C) and “cobble-stone” aspect) (D), presence bilaterally of infra-orbital cells (D), and very pneumatised sphenoid sinus (G).
Figure 11: (A-H) Coronal scans. Rounding of the supra-orbital recess (A,B), [ethmoidal emphysema of both sides but mainly on the left side, (ballooning lamina orbitalis and empty ethmoid - nearly no bony lamellae in the ethmioid itself)] very pneumatised bulla on the left (B,C,D) and on the right (E). Note different size between right and larger left ethmoid (E,F), waving of the orbital floor (E,F), and very extended sphenoidal sinus (G,H).
Figure 12: (A-C) Coronal scans of a 7 year old boy. Bilateral rounding of the supra-orbital recess (A,B,C), bilateral hyperpneumatised uncinate process (A,B), “ballooning” of lamina orbitalis (C), hyperinflated ethmoid “ethmoidal emphysema” with bilateral closure of the ethmoidal infundibulum resulting in bilateral chronic sinusitis.
Figure 13: (A-G) Coronal scans. Note invasion for more than 50 % of the frontal sinus (A,B) on the left side and of the supra-orbital (C) rim on the same side, resulting in bossing of the orbital rim (E) and a hyperinflated anterior ethmoidal complex (G).
Discussion
In the literature many signs of hyper inflated paranasal sinus structures have been described. However, most of these described hyper inflated structures concern the bony framework: [1-13]. Only a few of the anomalies of the ethmoid have been described such as: presence of the frontal cells in the frontal sinus [16,18], infra-orbital cells in the maxillary sinus [19], and pneumatisation of the uncinate process [20]. Several signs of hyper pneumatisation of the ethmoid bone are described for the first time in this article, such as “ballooning” and “cobble stone” appearance of the lamina orbitalis, ethmoidal emphysema (nearly empty ethmoid) and “egg shaped” ethmoid.
The pneumatisation of the facial bones is a continuous process that doesn’t stop after puberty. The life ongoing pneumatisation of the maxilla has been described already a very long time. Especially the dentition and the loss of dentition seem to be involved in the development of this sinus. Sometimes this pneumatisation can derail and result in EMSP [14] or a pneumosinus dilatans. Mostly, however, the signs of hyper pneumatisation are more discrete such as overdeveloped frontal or sphenoidal sinus, supra-orbital hyper pneumatisation of the frontal bone resulting in a rounding of the supra-orbital recess.
All the facial bones have in common that they are solid bony structures making up the frame-work of the face. The ethmoidal bone, on the contrary, is a unique bone. It is the only bone in the human body that forms very thin bony lamellae, thin bony cellular structures and thin bony conchae (media and superior). So it is not surprising that these thin delicate bony structures will be deformed more often than the thick bones of the facial skeleton. It is remarkable, however, that some of the deformations of the bony lamellae (“ballooning”, “cobble stone” and “egg-shaped” aspect of the lamina orbitalis) have never been described before.
Another unique feature of the ethmoidal bone is that it can invade other facial bones (extramural cells). More in particular its cellular structures can invade the space that has been created by the pneumatisation of the thick facial bones such as: frontal cells invading the frontal sinus and infra-orbital cells invading the maxillary sinus. The invasion of these facial bones by these very thin ethmoidal cells seems to be an in time dynamic process (Figures 2C-2D). From CT-scan studies it is obvious that the ethmoid has a tendency to expand outside its ZDFSVVVXVs. This expansion can be limited whenever a lot of resistance is encountered (orbital content stops the hyperextension of the lamina orbitalis resulting in “ballooning” and “cobble stone” aspect) or the extension can be more important when there is enough space available (frontal cells in the frontal sinus, infra-orbital cells in the maxillary sinus). Sometimes the expansion is towards the open space into the nasal cavity, blocking the ostiomeatal complex at the level of the middle meatus. When these hyperinflated structures are becoming too voluminous they will obstruct the narrow spaces of the ostiomeatal complex and interfere with proper ventilation and drainage of the involved sinuses, resulting in inflammation of the trapped mucosa. Frontal cells will induce chronic frontal sinusitis (Figure 2), hyper inflated ethmoid and uncinate process will close the infundibulum ethmoidale and obstruct the drainage of the maxillary sinus (Figure 12) or of the ostiomeatal complex at the level of the middle meatus resulting in a chronic sinusitis characterized on CT-scan by a “black halo” configuration (Figures 5,8).
One of the driving forces of the pneumatisation of the facial bones and the hyperinflation of the ethmoidal structures must be pressure. From table 1 [21] one can see that the maximum pressure generated by forced breathing are not very high (between 31 daPa and 75 daPa). The pressures, however, generated by nose blowing, are much higher (from 523 daPa to 950 daPa). The maximum positive pressure measured in this study reached even a level of 2420 daPa. One can imagine that such extreme high pressures can induce hyper pneumatisation of the facial bones and result into deformation and hyperinflation of the thin bony structures of the ethmoid. The amount of expansion will depend on one hand by the static and dynamic pressures generated during nose blowing manoeuvre and on the other hand by the yield or give way of the bony structures of the paranasal sinuses and the surrounding tissue. The static pressure will expand all sinuses in a similar way, the dynamic pressure will be directed mainly towards the anterior ethmoid complex via the hiatus semilunaris and the infundibulum ethmoidale towards the frontal and supra-orbital recess, maxillary and frontal sinus.
| Table 1: Maximum inspiratory pressures in daPa generated during normal nasal breathing. | ||
| Left side | Right side | |
| Control group mean n s.d. |
31 daPa 18 8 |
95 daPa 18 15 |
| Chronic rhinosinusitis mean n s.d. |
68 daPa 14 60 |
75 daPa 14 75 |
| Ref: Clement et al. [21] p=0.0028. | ||
| Table 2: Maximum pressures in daPa generated during nose blowing, both nostrils closed. | ||
| Left | Right | |
| Control group mean n s.d. |
620 daPa 18 208 |
533 daPa 18 183 |
| Chronic rhinosinusitis mean N s.d. |
860 daPa 14 495 |
950 daPa 14 577 |
| Ref: Clement et al. [21] p=0.015. | ||
From the results of the pressure measurements, it is obvious that patients with chronic sinusitis generate higher pressures than normal test subjects. Patients with chronic sinusitis not only blow their noses harder than normal test subjects, but they blow their noses also more frequently. So it might be possible that in a subgroup of patients with chronic sinusitis (excluding systemic diseases such as ASA, PCD and cystic fibrosis) bony deformities reach a size that will start to interfere with the drainage of the ostiomeatal complex [22] and sinuses.
Stammberger [20] described the “bottle neck” phenomenon where touching mucosae are responsible for an impaired mucociliary clearance, leading to chronic sinusitis. During a common cold, however, every patient experiences a severe blocked nose and examination of the nasal cavities shows presence of a swollen mucosa, blocking completely the middle meatus. Nevertheless a common cold seldom induces polyps. To generate chronic rhino sinusitis eventually with nasal polyps, the touching of the mucosae must be permanent and this is only possible when there exists an underlying bony deformity that closes off the narrow spaces of the ostiomeatal complex permenantly [22].
In recent literature a lot has been written on the role of fungae [23] and Staphylococcus aureus superantigens [24] in the pathogenesis of chronic rhino sinusitis. Recently, however, Bhattacharyya [25] was able to show no significant difference whatsoever in the positive culture rates of aerobic, anaerobic bacteriae and fungae between sides in patients with unilateral sinusitis. So this author concludes that there exists some doubt on the exact etiologic role of bacteria in chronic rhino sinusitis, suggesting other factors or other agents in the pathogenesis. Experimental studies show that to produce a bacterial biofilm in rabbits [26] one not only has to inoculate the maxillary sinus but one also has to block the maxillary ostium to be able to induce an experimentally induced chronic maxillary sinusitis in these animals. So impairment of the drainage is imperative in the production of a bacterial biofilm resulting in chronic sinusitis. The same goes for fungae. The fungal spores that are inhaled need at least one and a halve hour to germinate. This is only possible when the mucociliary transport is impaired.
All these data suggest that impaired mucociliary clearance of the ostiomeatal complex is still a very important factor in the etiology of chronic sinusitis [22]. Hyper inflated ethmoidal structures can impair mucociliary clearance and one can assume that high pressures generated during nose blowing are the most probable cause of hyper inflated bony structures blocking this clearance. During a common cold the frequency of nose blowing increases dramatically and one can imagine that the high pressures generated during forcefull nose blowing can be trapped in the sinuses because of a ball valve mechanism. Gwaltney et al. [17] showed that nose blowing can also blow pathological secretions in the sinuses (a kind of inoculation). So nose blowing does not only result in blocking the mucociliary drainage by expanding bony structures resulting in permanent obstruction of the narrow spaces of the ostiomeatal complex, but it can also inoculate the normally sterile paranasal sinuses of an adult. Both conditions i.e. blockage and inoculation that are necessary for an experimental induced rhino sinusitis can be generated by the nose blowing manoeuvre in patients. To avoid the generation of these extremely high pressures during nose blowing patients should be instructed to blow their noses one side at a time, without blocking both nostrils and building there extremely high pressures (Figure 14). Nose blowing is only useful when it is productive. When a nasal cavity is completely blocked by swollen mucosa nose blowing will only increase the mucosal swelling.
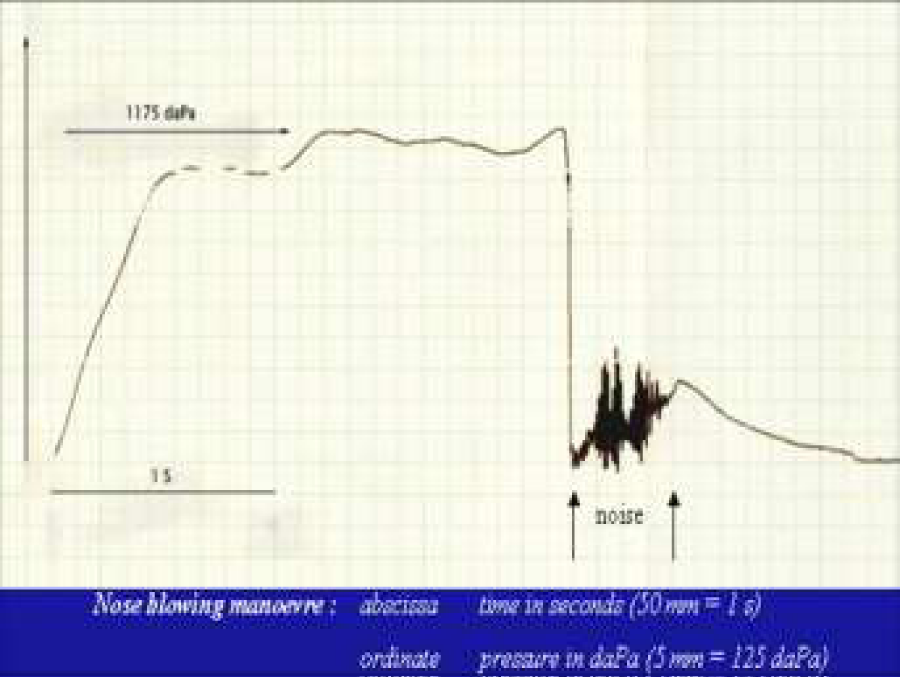
Figure 14: Pressure measurement in time of a nose blowing manoeuvre. Note the high pressure (1175 daPa) build up in a short time (0.5s) and the release of the pressure in 0.5 sec. while producing noise.
All the above described hyper inflated structures do also exist in healthy persons with no signs whatsoever of sinusitis. Chaiyasate et al. [27] studied twins without any sinus disease and in 40 % of the cases “cobblestone” aspect and in 38 % “ballooning” of the lamina orbitalis. In their study the two previous decscribed anomalies seemed to be rather acquired than from genetic origin.
It is very difficult to quantify these abnormalities. One can state that these oversized anomalies become pathological when the mucosa of the narrow spaces around these hyper inflated structures become inflamed.19 More studies are needed to evaluate the prevalence of these bony anomalies in normal test subjects and patients. Also the natural history of chronic sinusitis, from its early stage with discrete signs of sinusitis on the CT-scan but no or minimal complaints and normal endoscopy towards full blown chronic sinusitis, with obvious complaints and clear-cut signs of mucosal inflammation on CT-scan and endoscopy is poorly documented. Mostly the ENT surgeon sees these patients in a later stage, when medical therapy has failed and surgery is needed. The fact, however, remains that FESS alone can restore mucociliary clearance and ventilation of the paranasal sinuses and can induce healing in a major part of these patients, demonstrating that a simple obstruction of the ostiomeatal complex is still an important etiologic factor in the pathophysiology of chronic rhino sinusitis.
Conclusion
CT scans of patients with chronic sinusitis show many signs of hyper inflated paranasal sinus structures. It is possible that frequent and forced noseblowing could enhance these anomalies and as such may play a role in the pathophysiology of chronic rhino sinusitis.
References
- Lombardi G, Passerini A, Cecchini A. Pneumosinus dilatans. Acta Radiol Diagn (Stockh). 1968; 7: 535-542. Ref.: https://goo.gl/R5zxae
- Benjamins CE. Pneumo-sinus frontalis dilatans. Acta Otolaryngol (Stockh). 1918; 1: 414-423.
- Candan S, Muhtar H, Ciftçi A. Der Pneumosinus dilatans frontalis. Laryngol Rhinol Otol (Stuttg). 1990; 69: 552-533.
- Draf W, Constantinidis J, Weber R, Haque R. Pneumosinus dilatans frontalis. Etiology, symptoms and surgical technique. Laryngol Rhinol Otol (Stuttg). 1996; 75: 660-664.
- Klossek JM, Dufour X, Toffel P, Fontanel JP. Pneumosinus dilatans: A report of three new cases and their surgical management. Ear Nose Throat J. 2000; 79: 48-51. Ref.: https://goo.gl/FNMG7d
- Staudenmaier R, Happ T, Grevers G. Pneumosinus dilatans of the frontal sinus. HNO. 2002; 50: 480-182. Ref.: https://goo.gl/jMR2df
- Walker JL, Jones NS. Pneumosinus dilatans of the frontal sinuses: two cases and a discussion of its aetiology. J laryngol Otol. 2002; 116: 382-385. Ref.: https://goo.gl/GjL3od
- Wolfensberger M. Pathogenesis of pneumosinus maxillaris dilatans. HNO. 1984; 32: 518-520. Ref.: https://goo.gl/xT7ny4
- Sobin A, Carenfelt C, Haverling M, Anggard A. Pressure-induced expansion of the maxillary sinus. A rare entity. Rhinology. 1986; 24: 283-286. Ref.: https://goo.gl/ugrZtH
- Wolfensberger M, Herrmann P. The pathogenesis of maxillary sinus pneumoceles. Arch Otolaryngol Head Neck Surg. 1987; 113: 184-186. Ref.: https://goo.gl/XY62xU
- Mauri M, Oliveira de Oliveira C, France G. Pneumonsinus dilatans of maxillary sinus. Case report. Ann Otol Rhinol Larygol. 2000; 109: 278-280. Ref.: https://goo.gl/vnkdoz
- Juhl HJ, Buchwald C, Bollinger B. An extensive maxillary pneumosinus dilatans.
Rhinology. 2001; 39: 236-238. Ref.: https://goo.gl/uWR1CM - Ganly I, McGuiness R. Clinical problem solving: radiology: radiology quiz case 1. Pneumosinus dilatans of the maxillary, ethmoid, and sphenoid sinuses.
Arch Otolaryngol Head Neck Surg. 2002; 128: 1428-1430. Ref.: https://goo.gl/2LfK9R - Kalavagunta S, Reddy KT. Extensive maxillary sinus pneumatization. Rhinology. 2003; 41: 113-117. Ref.: https://goo.gl/YAehhi
- Neuner NT, Hausler R. Atypische Pneumatistions-former des Nasennebenhöhlensystems. Schweiz Med Wochenschr. 2000; 116: 108-112.
- Bent JP, Cuilty-Siller C, Kuhn FA. The frontal cell as a cause of frontal sinus obstruction. Am J Rhinology. 1994; 8: 185-191.
- Gwaltney JM Jr, Hendley JO, Phillips CD, Bass CR, Mygind N, et al. Nose blowing propels nasal fluid into the paranasal sinuses. Clin Infect Dis. 2000; 30: 387-391. Ref.: https://goo.gl/ycZifP
- Wormald PJ. Surgery of the frontal recess and frontal sinus. Rhinology. 2005; 43: 82-85. Ref.: https://goo.gl/xPRnm2
- Stackpole SA, Edelstein DR. The anatomic relevance of the Haller cell in sinusitis. Am J Rhinol. 1997; 11: 219-223. Ref.: https://goo.gl/LnNo8j
- Stammberger H, Kennedy DW, Anatomic Terminology Group. Paranasal sinuses: anatomic terminology and nomenclature. Ann Otol Rhinol Laryngol Suppl. 1995; 167: 7-16. Ref.: https://goo.gl/a8VzBD
- Clement P, Chovanova H. Pressures generated during nose blowing in patients with nasal complaints and normal test subjects. Rhinology. 2003; 41: 152-158. Ref.: https://goo.gl/rQzmKB
- Stackpole SA, Edelstein DR. Anatomic variants of the paranasal sinuses and their implications for sinusitis. Curr Opin Otolaryngol Head Neck Surg. 1996; 4: 1-11. Ref.: https://goo.gl/umkTBV
- Ponikau JU, Sherris DA, Kephart GM, Kern EB, Congdon DJ, et al. Striking deposition of toxic eosinophil major basic protein in mucus: implications for chronic rhinosinusitis. J Allergy Clin Immunol. 2005; 116: 362-369. Ref.: https://goo.gl/tx5eeP
- Bachert C, Gevaert P, Howarth P, van Cauwenberge P. Staphylococcus aureus enterotoxins: a key in airway disease? Allergy. 2002; 57: 480-487. Ref.: https://goo.gl/nFQRe7
- Bhattacharyya N. Bacterial infection in chronic rhinosinusitis: A controlled paired analysis. Am J Rhinol. 2002; 19: 544-548. Ref.: https://goo.gl/xnaCPY
- Perloff JR, Palmer JN. Evidence of bacterial biofilms in a rabbit model of sinusitis. Am J Rhinol. 2005; 19: 1-6. Ref.: https://goo.gl/ra5k5A
- Chaiyasate S, Baron I, Clement P. Analysis of paranasal sinus development and anatomical variations: a CT genetic study in twins. Clin Otolaryngol. 2007; 32: 93-97. Ref.: https://goo.gl/PaBPDh